Review
Abstract
Historically, antiobesity medications have been modestly effective at best, with side-effect profiles that limit compliance and often preclude the long-term therapy required to maintain weight loss. Recently developed therapies based on analogs of the gut hormone glucagon-like peptide-1 (GLP-1) have transformed the medical management of obesity, leading both to a degree of weight loss that rivals bariatric surgery and a reduction in morbidity and mortality associated with obesity-related complications. GLP-1 receptor agonist (GLP-1RA) therapies were developed to mimic the peripheral effects of GLP-1, but it is now well established that their efficacy in the treatment of obesity depends on reducing energy intake through their action in the central nervous system (CNS). Recent data indicate that the aversive gastrointestinal side effects of GLP-1RAs are also CNS mediated. Although a complete understanding of the neural circuits underlying GLP-1RA–induced weight loss remains elusive, a great deal has been learned in recent years. This Review summarizes proposed gut-brain and central mechanisms through which GLP-1 and its synthetic analogs regulate food intake and bodyweight.
Authors
Lisa R. Beutler
Abstract
The glucagon-like peptide-1 receptor (GLP-1R) is a class B1 G protein–coupled receptor and major therapeutic target in type 2 diabetes and obesity. Beyond its canonical role in Gαs/cAMP signaling, GLP-1R is increasingly recognized as an organizer of spatiotemporally defined signaling nanodomains, or “signalosomes.” This Review highlights our current knowledge on the mechanisms of assembly and regulation of GLP-1R signalosomes, including the involvement of biomolecular condensates formed by liquid-liquid phase separation, and the role of membrane contact sites between the endoplasmic reticulum (ER) and other organelles as key locations for GLP-1R signaling assemblies. Furthermore, we discuss existing data on the molecular composition and functional impact of two predicted GLP-1R nanodomains, one at ER–plasma membrane contact sites, where GLP-1R might interact with ion channels and transporters to influence local excitability and coordinated insulin secretion, and another at ER–mitochondria membrane contact sites, with the capacity to control lipid and calcium signaling and modulate ER and/or mitochondrial activity. We additionally discuss the role of GLP-1R posttranslational modifications as critical modulators of GLP-1R signal specification and nanodomain organization. Conceptualizing GLP-1R as a dynamic architect of spatiotemporally encoded signalosomes opens new avenues for a deeper understanding of incretin biology with the potential for identification of novel GLP-1R effectors and the development of refined therapeutic strategies for metabolic disease.
Authors
Gregory Austin, Alejandra Tomas
Abstract
Connections between the digestive system and the brain have been postulated for over 2000 years. Despite this, only recently have specific mechanisms of gut-brain interaction been identified. Due in large part to increased interest in the microbiome, the wide use of incretin-based therapies (i.e., glucagon-like peptide 1 [GLP-1] receptor agonists), technological advancements, increased understanding of neuroimmunology, and the identification of a direct enteroendocrine cell–neural circuit, research in the past 10 years has made it abundantly clear that the gut-brain connection plays a role both in clinical disease as well as the actions of therapeutics. In this Review, we describe mechanisms by which the gut and brain communicate and highlight human and animal studies that implicate changes in gut-brain communication in disease states in gastroenterology, neurology, psychiatry, and endocrinology. Furthermore, we define how GLP-1 receptor agonists for obesity and guanylyl cyclase C agonists for irritable bowel syndrome leverage gut-brain mechanisms to improve patient outcomes. This Review illustrates the critical nature of gut-brain communication in human disease and the potential to target gut-brain pathways for therapeutic benefit.
Authors
Zachary S. Lorsch, Rodger A. Liddle
Abstract
The urokinase plasminogen activator receptor (uPAR) is a membrane-bound protein found on the surface of immune cells. Through the action of proteases, uPAR is cleaved to produce several circulating proteins in the bloodstream, including the soluble form suPAR and the fragments D1 and D2D3. Initially studied in the context of infectious diseases and cancer, recent research has revealed roles for suPAR and its related proteins as mediators linking innate immunity to the pathogenesis of kidney and cardiovascular diseases, as well as insulin-dependent diabetes. While these proteins have long been recognized as prognostic biomarkers, growing clinical, experimental, and genetic evidence highlights their active involvement in the onset and progression of these diverse conditions. This Review examines suPAR’s evolution from its discovery as a modulator of innate immunity to its current status as a key driver in chronic kidney and cardiovascular diseases. Furthermore, we explore the molecular mechanisms through which suPAR and D2D3 contribute to multiorgan damage, emphasizing emerging opportunities for therapeutic interventions across interconnected organ systems.
Authors
Jochen Reiser, Salim S. Hayek, Sanja Sever
Abstract
The maternal cardiovascular system undergoes dramatic remodeling in response to the stresses of pregnancy. Although in most cases these changes are temporary and well tolerated, in others they can give rise to complications, including cardiomyopathy, coronary artery disease, and hypertensive cardiovascular disease. Despite an increasing number of preclinical models to study these diseases, specific treatments for any of these pregnancy complications are lacking. As the maternal mortality rate is rising in the United States, it is critical to understand the molecular mechanisms driving cardiovascular changes during pregnancy, and the pathology that can result.
Authors
Yijun Yang, Jennifer Lewey, Zoltan Arany
Abstract
The renin-angiotensin-aldosterone system (RAAS) is a central regulator of cardiovascular, renal, and fluid homeostasis. Over the past century, our understanding of RAAS has evolved from a unidimensional circulatory hormone system to a complex network that includes local and intracellular signaling pathways. Aging profoundly impacts this system, influencing both systemic and tissue-specific RAAS activity. While levels of systemic RAAS components, such as plasma renin and aldosterone, decline with age, local RAAS components, particularly the proinflammatory angiotensin (Ang)II/AngII type 1 receptor (AT1R) axis, are upregulated in aging tissues, contributing to vasoconstriction, oxidative stress, inflammation, and fibrosis. Conversely, the protective arms of RAAS, the AngII/AT2R and Ang-(1–7)/Mas receptor pathways, are downregulated. Recent advances in geroscience have further illuminated how RAAS intersects with fundamental aging mechanisms, providing a mechanistic framework for understanding RAAS not only as a driver of age-related disease but also as a modifiable contributor to the aging process itself. In this Review, we summarize the evolution of RAAS biology, examine the molecular and functional consequences of aging on RAAS activity, and discuss the translational relevance of these findings. Finally, we explore emerging therapeutic strategies targeting RAAS components as potential interventions to promote healthy aging and reduce age-related disease burden, emphasizing a translational arc moving from bedside to bench and back, with the ultimate goal of improving patient outcomes.
Authors
Caglar Cosarderelioglu, Peter M. Abadir
Abstract
Therapies based on glucagon-like peptide-1 (GLP-1) reduce rates of cardiovascular and chronic kidney disease in people with type 2 diabetes and/or obesity, with ongoing clinical trials investigating their effects in people with metabolic liver disease, arthritis, and both substance use and neurodegenerative disorders. Acute and chronic activation of GLP-1 receptor signaling also reduces systemic and tissue inflammation in mice and humans, through weight loss–dependent and –independent mechanisms, actions that may contribute to the expanding spectrum of clinical benefits ascribed to GLP-1 medicines. In this Review, we highlight current understanding of the direct and indirect antiinflammatory effects and mechanisms of GLP-1 medicines in both preclinical and clinical studies, covering emerging concepts, clinical relevance, and areas of uncertainty that require further investigation.
Authors
Chi Kin Wong, Daniel J. Drucker
Abstract
Glucagon-like peptide-1 (GLP-1) was initially considered to be a hormone with a predominant role in regulating glucose metabolism by inducing insulin secretion, reducing glucagon secretion, and ameliorating insulin resistance, with the last effect being largely dependent on the induction of weight loss. In more recent years, the role of this peptide beyond metabolism has progressively been explored, including its impact on kidney physiology and kidney clinical outcomes in people with obesity with or without diabetes. Indeed, despite only modest expression of the GLP-1 receptor in the kidney, the renoprotective actions of GLP-1 and its receptor agonists have become an area of intensive investigation. This Review appraises the current status of GLP-1 peptide and its receptor agonists and focuses on the preclinical as well as recent seminal clinical findings defining the kidney benefits conferred by GLP-1 receptor agonist treatment in people living with type 2 diabetes and obesity.
Authors
Mark E. Cooper, Daniël H. van Raalte
Abstract
Cancer diagnoses are prevalent in people with obesity and type 2 diabetes, and abundant clinical evidence supports the protective effects of weight loss for cancer prevention. Glucagon-like peptide-1 (GLP-1) receptor agonists have revolutionized obesity and type 2 diabetes medicine and alleviate many comorbidities of these metabolic diseases. In this Review, we summarize the current clinical evidence for GLP-1 receptor agonists and cancer risk, including thyroid, pancreatic, gastrointestinal, and hormone-dependent malignancies. With few exceptions, recent meta-analyses report that GLP-1 receptor therapies do not increase cancer incidence and may lower risk in some cases. Preclinical studies reinforce the anticancer effects of GLP-1 receptor therapies, even in non-obese models. However, there are still many opportunities for translational insight as the field grows. Immune-modulating effects of GLP-1 receptor agonists are reported in several preclinical cancer studies, which may reflect direct action on immune cells or result from improved metabolic function. We highlight ongoing clinical trials for GLP-1 receptor therapies in cancer patients, and offer considerations for preclinical studies, including perspectives on the timing and duration of GLP-1 receptor agonist treatment, concurrent use of standard anticancer therapies, and interpretation of models of cancer risk versus progression.
Authors
Estefania Valencia-Rincón, Rajani Rai, Vishal Chandra, Elizabeth A. Wellberg
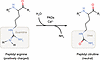
Abstract
Peptidyl arginine deiminases (PADs) catalyze the conversion of arginine residues into peptidyl citrulline, a posttranslational modification known as protein citrullination (or arginine deimination). This process alters the charge of proteins from positive to neutral, thereby affecting their folding, stability, conformation, and function. PAD2 and PAD4 can translocate into the nucleus and citrullinate both cytoplasmic and nuclear proteins. In this Review, we focus on PAD2- and PAD4-mediated citrullination in immune cell subsets within the tumor microenvironment. We discuss how citrullination regulates immune cell function and tumor immunity and explore the potential of targeting citrullination as a strategy for cancer immunotherapy.
Authors
Michael R. Pitter, Weiping Zou
No posts were found with this tag.