Advertisement
Review
Open Access |  10.1172/JCI194018
10.1172/JCI194018
Perturbation of the circadian clock in chronic diseases involving organ fibrosis
Atish Mukherji,1 Pierre-Louis Tharaux,2 David W. Ray,3,4,5 and Thomas F. Baumert1,6,7,8
1University of Strasbourg, Institute of Translational Medicine and Liver Diseases (ITM), Inserm UMR_S1110, Strasbourg, France.
2Université Paris Cité, Inserm, Paris Cardiovascular Centre–PARCC, Paris, France.
3National Institute for Health and Care Research (NIHR), Oxford Biomedical Research Centre, John Radcliffe Hospital, Oxford, United Kingdom.
4Oxford Centre for Diabetes, Endocrinology and Metabolism and
5Oxford Kavli Centre for Nanoscience Discovery, University of Oxford, Oxford, United Kingdom.
6Gastroenterology and Hepatology Service, Strasbourg University Hospitals, Strasbourg, France.
7Institut Universitaire de France, Paris, France.
8Institut Hospitalo-Universitaire (IHU) Strasbourg, Strasbourg, France.
Address correspondence to: Thomas F. Baumert or Atish Mukherji, Inserm U1110, University of Strasbourg, 3 Rue Koeberlé, 67000 Strasbourg, France. Phone: 33.3.68.85.37.03; Email: thomas.baumert@unistra.fr (TFB); mukherji@unistra.fr (AM).
Find articles by Mukherji, A. in: PubMed | Google Scholar
1University of Strasbourg, Institute of Translational Medicine and Liver Diseases (ITM), Inserm UMR_S1110, Strasbourg, France.
2Université Paris Cité, Inserm, Paris Cardiovascular Centre–PARCC, Paris, France.
3National Institute for Health and Care Research (NIHR), Oxford Biomedical Research Centre, John Radcliffe Hospital, Oxford, United Kingdom.
4Oxford Centre for Diabetes, Endocrinology and Metabolism and
5Oxford Kavli Centre for Nanoscience Discovery, University of Oxford, Oxford, United Kingdom.
6Gastroenterology and Hepatology Service, Strasbourg University Hospitals, Strasbourg, France.
7Institut Universitaire de France, Paris, France.
8Institut Hospitalo-Universitaire (IHU) Strasbourg, Strasbourg, France.
Address correspondence to: Thomas F. Baumert or Atish Mukherji, Inserm U1110, University of Strasbourg, 3 Rue Koeberlé, 67000 Strasbourg, France. Phone: 33.3.68.85.37.03; Email: thomas.baumert@unistra.fr (TFB); mukherji@unistra.fr (AM).
Find articles by
Tharaux, P.
in:
PubMed
|
Google Scholar
|

1University of Strasbourg, Institute of Translational Medicine and Liver Diseases (ITM), Inserm UMR_S1110, Strasbourg, France.
2Université Paris Cité, Inserm, Paris Cardiovascular Centre–PARCC, Paris, France.
3National Institute for Health and Care Research (NIHR), Oxford Biomedical Research Centre, John Radcliffe Hospital, Oxford, United Kingdom.
4Oxford Centre for Diabetes, Endocrinology and Metabolism and
5Oxford Kavli Centre for Nanoscience Discovery, University of Oxford, Oxford, United Kingdom.
6Gastroenterology and Hepatology Service, Strasbourg University Hospitals, Strasbourg, France.
7Institut Universitaire de France, Paris, France.
8Institut Hospitalo-Universitaire (IHU) Strasbourg, Strasbourg, France.
Address correspondence to: Thomas F. Baumert or Atish Mukherji, Inserm U1110, University of Strasbourg, 3 Rue Koeberlé, 67000 Strasbourg, France. Phone: 33.3.68.85.37.03; Email: thomas.baumert@unistra.fr (TFB); mukherji@unistra.fr (AM).
Find articles by Ray, D. in: PubMed | Google Scholar
1University of Strasbourg, Institute of Translational Medicine and Liver Diseases (ITM), Inserm UMR_S1110, Strasbourg, France.
2Université Paris Cité, Inserm, Paris Cardiovascular Centre–PARCC, Paris, France.
3National Institute for Health and Care Research (NIHR), Oxford Biomedical Research Centre, John Radcliffe Hospital, Oxford, United Kingdom.
4Oxford Centre for Diabetes, Endocrinology and Metabolism and
5Oxford Kavli Centre for Nanoscience Discovery, University of Oxford, Oxford, United Kingdom.
6Gastroenterology and Hepatology Service, Strasbourg University Hospitals, Strasbourg, France.
7Institut Universitaire de France, Paris, France.
8Institut Hospitalo-Universitaire (IHU) Strasbourg, Strasbourg, France.
Address correspondence to: Thomas F. Baumert or Atish Mukherji, Inserm U1110, University of Strasbourg, 3 Rue Koeberlé, 67000 Strasbourg, France. Phone: 33.3.68.85.37.03; Email: thomas.baumert@unistra.fr (TFB); mukherji@unistra.fr (AM).
Find articles by Baumert, T. in: PubMed | Google Scholar
Published October 1, 2025 - More info
J Clin Invest. 2025;135(19):e194018. https://doi.org/10.1172/JCI194018.
© 2025 Mukherji et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
-
Abstract
Chronic organ disease is often complicated by fibrosis, the excessive accumulation of extracellular matrix, as a consequence of dysfunctional wound healing responses. Fibrosis progressively distorts tissue architecture and eventually leads to loss of organ function, accounting for up to 45% of deaths in developed countries. Moreover, fibrosis is a major risk factor for tumor development. The few approved therapies aimed at preventing or resolving fibrosis show limited efficacy and safety. One reason for the lack of efficient antifibrotic therapies is the fact that the cell circuits driving the disease biology are still only partially understood. The circadian clock is known to regulate the physiological functions of critical organs, including the liver, kidneys, and lungs. Several experimental and clinical studies have established that circadian disruption plays an important role in the development of chronic diseases across organs involving fibrosis. These include metabolic dysfunction–associated steatotic liver disease, chronic kidney disease, and chronic obstructive pulmonary disease. Here, we provide an overview of the circadian mechanisms that play critical roles in mediating physiological functions in the liver, kidneys, and lungs and whose deregulations could predispose toward development of chronic disease of these organs, leading to fibrosis. We also highlight the possible opportunities of chronotherapy for chronic diseases and discuss future perspectives.
-
Introduction to the circadian clock: principles and components
Iconic solar worship sites in different continents suggest that humanity has been conscious of a world dominated by the daylight cycle throughout history. Most terrestrial life forms display biological rhythms with a period of approximately 24 hours, allowing them to prepare for daily variations of the light-dark cycle. This endogenous rhythm is known as a circadian rhythm, and it coordinates the physiology of organisms with the light cycle and behaviors, including feeding-fasting and sleep-wake cycles (1–6). Discovery of the first circadian gene in Drosophila initiated the molecular clock revolution (7). Mammalian circadian rhythms require periodic entrainment to remain synchronized with different “zeitgebers,” or time cues, e.g., the light-dark cycle (8–12). This synchronization process relies on light information from the retina passing to the hypothalamic suprachiasmatic nucleus, which is the site of the central circadian clock. This central clock then disseminates signals to the clocks in most cells of the body, ensuring they oscillate in phases (8–12) (Figure 1).
 Figure 1
Figure 1Circadian regulation of the cellular clock and physiological outputs. The central circadian clock in the suprachiasmatic nucleus (SCN) of the hypothalamus synchronizes peripheral clocks. Within each organ, every cell contains a circadian clock (CC) oscillator, based on a negative transcription-translation feedback loop, that drives expression of numerous clock-controlled genes (CCGs). The CC oscillators in different cell types are largely responsible for maintaining essential physiological functions. ERAD, endoplasmic reticulum–associated degradation; FEV1, forced expiratory volume in 1 second; GFR, glomerular filtration rate; PEF, peak expiratory flow; VE, pulmonary ventilation; VO2, oxygen consumed; VCO2, carbon dioxide exhaled.
The cellular circadian clock (CC) runs as a transcription-translation feedback loop and is conserved across cell types (12–14) (Figure 1). The core of the CC oscillator is made up of two transcription factors: CLOCK (or its paralog NPAS2) and its partner BMAL1. In the rest phase (night for humans), the BMAL1-CLOCK complex is recruited to E-box motifs present in the regulatory regions of several genes, including the repressor families Period (PER1, PER2) and Cryptochrome (CRY1, CRY2) (12–14) (Figure 1). In the late rest phase, phosphorylated PER and CRY proteins heterodimerize to repress the activity of BMAL1-CLOCK, inhibiting their own expression (12–14). Additionally, BMAL1-CLOCK activates transcription of REV-ERBa and REV-ERBb, which in complex with HDAC3 are recruited to inhibit expression of RORE-containing genes, e.g., BMAL1 and CLOCK (13–18). In active phase, RORα/γ activates BMAL1 and CLOCK transcription, enabling the start of the next cycle (12–14, 19, 20) (Figure 1). Together, these mechanisms generate rounds of rhythmic transcription at different phases depending on the combination of DNA-binding elements (13–16). Depending on the organ/tissue, 10%–20% of the genome is transcribed rhythmically (21–27). The CC also influences splicing, mRNA polyadenylation, mRNA export, and translation (5, 13). Importantly, distinct circadian rhythms can persist in the absence of core clock gene expression through mechanisms that remain poorly understood. Additionally, peripheral clocks can be entrained by non-photic stimuli, notably food timing (28–32).
Teleologically, zeitgebers are required to maintain the near-24-hour rhythms to optimize physiological adaptation (1–3, 12, 30). Circadian misalignment occurs when our internal rhythm is out of phase with the natural light-dark cycle (1–3, 30). Examples include jet lag and shift work, which are associated with increased risk of chronic diseases, including diabetes, metabolic syndrome, cardiovascular disorders, and renal disease, as well as fibrosis and cancer (23–27, 30, 33–35) (Figure 2).
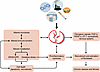 Figure 2
Figure 2The perturbed “clock” as a candidate driver of chronic disease leading to fibrosis and organ failure. The model depicts a simplified global view of how various events (e.g., genetics, lifestyle, environment, habits) perturb the clock activity, which in turn alters multiple functions and pathways, leading to the development of chronic disease and fibrosis. The left side of the figure shows the bidirectional communication between the clock machinery and metabolism, microbiota signaling, and immune functions. This communication regulates cellular functions (such as ERAD/UPR, cytokine production, cell death, and oxidative stress) in a temporal manner, thereby maintaining homeostasis. As shown on the right side, perturbation of the clock disrupts metabolism, triggers activation of the immune system, and elevates stress responses, driving chronic diseases and fibrosis. Notably, mesenchyme-derived fibroblasts of different subtypes are known to harbor functional cellular clocks, which are disrupted in chronic disease, leading to deregulated production of key mediators and effectors of fibrosis. ERAD, endoplasmic reticulum–associated degradation; ET-1, endothelin-1; UPR, unfolded protein response.
Importantly, circadian rhythm disruption is also seen in early life (36, 37). Investigations in mouse models have shown that chronodisruption affects physiology, development, and growth in both pre- and postnatal life (36–39). Epidemiological studies indicate that circadian disruption during pregnancy via shift work is associated with adverse outcomes at birth (miscarriage, preterm delivery) and later in life (such as sleep disorder, bipolar disorder, susceptibility to infections, aging) (36–39).
Almost all chronic diseases induced by circadian perturbation, such as diabetes, metabolic syndrome, and inflammation, ultimately result in structural remodeling of their tissues through fibrosis. Thus, an understanding of how CC perturbation contributes to fibrosis during chronic metabolic or inflammatory disease is important in order to understand disease biology and therapeutic opportunities.
-
Fibrosis: underlying common principles and molecular drivers
Fibrosis of solid organs is a major cause of morbidity and mortality worldwide. Advanced fibrosis ultimately leads to organ failure or cancer (40–44). Globally, fibrosis has emerged as a leading contributor to disease burden, affecting nearly 5,000 patients for every 100,000 cases (40–45). Despite large research and development efforts, antifibrotics for the kidney are lacking and have limited therapeutic efficacy in lungs and liver. Since the mechanisms underlying fibrosis have been extensively reviewed (40–55), here we provide a brief overview of common concepts across organs relevant to understanding their interplay with the CC.
Fibrosis is characterized by the accumulation of extracellular matrix (ECM) proteins in tissues, which distort their architecture and perturb their physiological functions (40–44). Fibrosis is not a disease per se but rather an outcome of the tissue’s reparative response to chronic injury. While the ability to repair wounds successfully is advantageous, excess matrix deposition in chronic disease states is detrimental (43, 46, 51, 55). This maladaptive accumulation of ECM can be triggered by multiple factors, e.g., viral or bacterial pathogens, high-fat diets, alcohol, smoking, drug toxicity, air pollutants, diabetes, and genetic mutations (43, 46, 51, 53–55) (Figure 2).
The fibrotic response comprises multiple stages (Figure 3). Typically, injury of epithelial cells leads to inflammation and initiates fibrosis (42, 46, 56–59). Inflammation recruits mesenchymal-origin cells, mainly fibroblasts, e.g., hepatic stellate cells (HSCs) and alveolar fibroblasts, to the injured parts (42, 46, 56–59). Next, increased expression of fibrosis-driving cytokines, including TGF-β, FGFs, and PDGFs, drives differentiation of fibroblasts to myofibroblasts (42, 46, 60–63) (Figure 3). The TGF-β signaling cascade plays a key role in fibrosis. In healthy tissues, the TGF-β level is minimal. However, upon tissue damage, TGF-β expression increases, which interacts with TGFBRs, resulting in SMAD2 and SMAD3 activation. Next, SMAD2 and SMAD3 heterodimerize with SMAD4 and transcriptionally activate the expression of several profibrotic genes, including collagens (60–63). Additionally, TGF-β induces SMAD-independent pathways to augment fibrotic gene expression (60–63). Altogether, activation of these pathways leads to enhanced production of ECM remodelers (collagens, fibronectins, basement membrane proteins, and α-smooth muscle actin) (40–43, 46). ECM remodeling enhances tissue stiffness, reduces oxygen diffusion (elevating oxidative and hypoxic stress), and eventually compromises organ function and cell death, thereby perpetuating and aggravating the fibrotic damage (40–43, 46–53) (Figure 3).
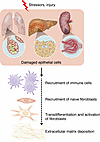 Figure 3
Figure 3Common steps in organ fibrosis. Multiple metabolic and inflammatory stressors and injury damage epithelial cells in different organs, initiating the pathogenic cascade of fibrosis. In chronic disease, damaged epithelial cells raise an evolutionarily conserved protective response involving multiple cell types. Briefly, epithelial damage leads to attraction of immune cells, which secrete multiple factors to enable recruitment of naive fibroblasts of mesenchymal origin. Once recruited into this inflammatory milieu, fibroblasts are transdifferentiated and activated. With FGF, TGF-β, and PDGF-β as key mediators, fibroblasts produce and secrete multiple collagens, which increase stiffening and remodel the extracellular matrix.
-
Circadian drivers of metabolic liver disease and fibrosis
The circadian clock and liver physiology. A healthy liver is critical for maintaining metabolic homeostasis, and the liver CC plays a pivotal role in this process. Studies in mouse livers revealed rhythmic mRNA accumulation for approximately 10%–15% of the genome (13, 21, 28, 29, 64). This rhythmicity in hepatic gene expression largely arises from circadian phase–specific DNA binding of CC genes and clock-regulated transcription factors (13, 64–69). Hepatic circadian transcription involves changes in three-dimensional genome and has implicated a role for REV-ERBα (70–72). Hepatic gene expression is also controlled by zonation, or spatial positioning relative to the liver’s central vein and peripheral portal tracts (73). A combination of single-cell RNA sequencing with FISH revealed that several key metabolic genes are controlled by both zonation and the liver CC (74). Importantly, in mice with hepatocyte-specific knockout of the CC genes Rev-erba and Rev-erbb, single-nucleus RNA sequencing revealed that the hepatocytic clock dictates cellular communication in liver by regulating gene expression in non-parenchymal cells such as liver endothelial cells and Kupffer cells (75). Interestingly, HSCs have also been shown to control zonation and liver function (76). Lipidomic, proteomic, and metabolomic studies have confirmed the widespread role of the CC oscillator and meal timing in dictating liver physiology (32, 77–81).
Since the role of the CC system in regulating liver metabolism has been reviewed (4, 5, 23, 33–34), here we summarize key features that, when deregulated, contribute to chronic metabolic liver disease resulting in fibrosis (Table 1). The liver CC controls blood glucose levels by regulating both preprandial gluconeogenesis and postprandial glycogen synthesis (4, 5, 23, 82). The liver CC controls glucose metabolism by regulating expression of key genes, e.g., glucokinase (Gck), phosphoenolpyruvate carboxykinase (Pck1), and glucose transporter 2 (Glut2) (4, 5, 23, 82). The CC also influences the glucoregulatory transcriptional activity of CREB, CHREBP, and GR (23, 82–84). Regarding lipid metabolism, plasma triglycerides (TGs), free fatty acids (FFAs), and cholesterol display circadian variations (4, 5, 23, 33, 34) and are disrupted following knockout of CC genes such as Clock (85), Rev-Erba, and Rev-Erbb (86, 87). The liver CC regulates hepatic TG levels by controlling the expression of enzymes such as Gpat2, Lipin1/2, and Dgat2, while REV-ERBα represses Insig2 and miR122 levels to regulate SREBP1c, the driver of hepatic de novo lipogenesis (4, 5, 23, 88). The metabolism of FFAs, bile acids, and xenobiotics is also controlled by the CC (4, 5, 23). The liver CC also controls cellular processes such as endoplasmic reticulum (ER) stress, unfolded protein response (UPR), autophagy, and response to reactive oxygen species (ROS), all of which are indispensable for metabolic homeostasis (4, 5, 82, 89–91) (Figure 1).
 Table 1
Table 1Contribution of CC perturbation in different organs and cell types in the development of chronic diseases resulting in fibrosis
Recent studies also investigated the CC in myofibroblasts. Using enriched murine HSCs and human myofibroblasts, the existence of CC genes and their circadian expression were demonstrated (92). Furthermore, this study identified nearly 2,000 rhythmically expressed genes involved in metabolism, stress response, collagen/ECM synthesis, and cell cycle in HSCs (92). Among the rhythmically expressed HSC genes were members of the TGF-β/BMP/activin pathways, including Smad3, Smad7, Smad6, receptors (Acvr1, Bmpr1a, Tgfbr1), and ligands (Bmp2, Bmp3, Tgfb1) (92). Future studies (e.g., applying fibroblast-CC mutant mouse models) will be essential to decipher the importance of dynamic circadian communication between different liver cell types in health and disease.
Although the circadian biology of the mouse liver is well studied, the identity of rhythmic genes in human hepatocytes is mostly unknown owing to challenges associated with collecting multiple biopsies over 24 hours. Recent studies (93, 94) performed 24-hour transcriptomic analyses using humanized liver chimeric mice (HLCM) as a surrogate for human liver (95, 96). These investigations unraveled the human hepatocytic circadian transcriptome, including common and distinct rhythmic genes and biochemical processes. These studies also revealed rhythmic pathways shared and discordant between human and mouse hepatocytes (93, 94). Notably, genes metabolizing carbohydrates, FFAs, TGs, and bile acids were shown to display rhythmicity in both human and mouse hepatocytes (94).
Disruption of the liver clock, metabolism, and fibrosis. Lifestyle changes and effective antiviral therapies have largely shifted the major causes of liver disease from viral to metabolic diseases. Owing to the global epidemic of obesity, metabolic dysfunction–associated steatotic liver disease (MASLD; formerly NAFLD) is currently emerging as the most prevalent chronic liver disease (CLD), affecting 20%–25% of the world population (97–100). MASLD is the liver manifestation of metabolic syndrome. MASLD encompasses a wide range of conditions, from benign metabolic dysfunction–associated steatotic liver (MASL) to metabolic dysfunction–associated steatohepatitis (MASH), a risk factor for developing hepatocellular carcinoma (HCC) (97–100). Modern diets characterized by overconsumption of energy-dense foods and fructose-containing drinks are key for driving metabolic syndrome. Both systemic and tissue-specific metabolic deregulation leads to hepatic stress and cell death, thereby creating an inflammatory milieu that eventually initiates fibrotic responses (97–100) (Figure 2).
Fibrosis is the major determinant of clinical outcomes in MASH and is associated with increased risk of cirrhosis and HCC (98, 99). In the healthy liver, HSCs are largely non-proliferative. However, liver injury leads to activation of HSCs, driving their differentiation to myofibroblasts, which are proliferative and contractile and upregulate expression of α-smooth muscle actin (αSMA) and multiple collagens. HSC activation results from several signals, such as proinflammatory cytokines, apoptotic hepatocytes, and increased ROS production (48–50, 57, 58). Activated HSCs increase the secretion of profibrotic molecules (TGF-β and PDGF-β), which enable further recruitment of immune cells (48–50, 57, 58). Thus, CLD-induced HSC activation eventually disrupts liver architecture and functions.
Interestingly, the perturbation of the crosstalk between metabolism and CC drives different liver pathologies, including MASLD. MASLD arises due to energy surpluses created by (a) alterations in glucose and FFA metabolism, (b) increased de novo lipogenesis, and (c) compromised β-oxidation or reduced hepatic TG exports (100). TG accumulation in hepatocytes increases ER stress, UPR, and ROS levels, which together act as triggers for MASLD and its sequela, fibrosis, by recruiting immune cells (100, 101). Importantly, metabolic perturbations can alter the functioning of both the hepatic and myofibroblast CC oscillators (5, 33, 34, 92). Circadian misalignment driven by daytime-restricted feeding or jet lag drives liver CC perturbation (102, 103). Meal timing also affects liver proteome, phosphoproteome, and lipidome (32). MASLD features, including increased TG levels and fibrosis, have been noted in mice fed with different versions of high-fat diet as well as with changes in meal timing (23, 27, 32, 34, 35). MASLD breaks down the HSC CC oscillator, and this correlates with elevated expression of profibrotic collagens and αSMA (92) (Table 1). Furthermore, disruption of CC functioning induced by genetic mutations of Clock and Rev-Erbs leads to liver steatosis (85–87), while absence of Bmal1 in hepatocytes is known to predispose to HCC (104). Finally, not only metabolic but also infectious diseases perturb the rhythmic transcriptome in human hepatocytes, and predispose to CLD, fibrosis, and HCC, as shown for chronic hepatitis C virus infection (94).
-
Circadian disruption in chronic kidney disease and fibrosis
Circadian clock and renal physiology. Kidneys filter blood and maintain fluid and electrolyte balance, which contributes to sustaining blood pressure (BP) (105–111). Circadian variation in renal functions has been observed in several species (112–116), including the circadian rhythmicity of urinary excretion of sodium, potassium, chloride, and phosphate (117, 118). In humans, urinary volume and pH also display 24-hour rhythmicity under relatively constant conditions of eating, drinking, and sleeping (119). Circadian rhythms also exist for the glomerular filtration rate (GFR), corticomedullary osmotic changes, blood flow, and transport of water and electrolytes, which are believed to be driven in part by the kidney CC (105–110). Global disruption of Bmal1 in rats revealed a sex-dependent dissociation between circadian BP variation and control of sodium excretion, as only female Bmal1–/– rats had significantly greater sodium excretion during the active phase compared with controls (120). Additionally, murine kidney-specific cadherin-Cre–mediated (Ksp-Cre–mediated) Bmal1 knockout in distal tubules suggests its role in BP control and Na+ handling in response to a K+-depleted diet, but only in male mice (121).
Kidneys and their functional units, nephrons, comprise over 30 different cell types that contribute to renal physiology. The existence of a “kidney clock” has been noted in rats and mice during embryonic development (122–124). Analyses from mouse fetal kidney (E18–E20) detected thousands of rhythmic transcripts, which included cell cycle and DNA repair genes, drivers of nephron development (Hoxb7 and Pax2), epithelial sodium channel α subunit (Scnn1a, encoding αENaC), and sodium/hydrogen exchanger (Slc9a3) (105–110). Investigations using global-Bmal1-KO and ureteric bud–specific (Hoxb7 Cre-Bmal1-KO) mutant mice have revealed the role of CC in controlling nephron development. RNA sequencing from kidneys of adult mice indicated that approximately 13% of the genome (second only to what is observed in liver) is expressed in a circadian manner, suggesting a crucial role in driving renal physiology. Transcriptomic studies from different kidney parts, e.g., distal convoluted tubule, connecting tubule, and cortical collecting duct, not only revealed cell-intrinsic rhythmic expression of key CC genes but also identified numerous genes (involved in water and electrolyte balance, BP, and metabolic processes) that display circadian expression and were disrupted in Clock-KO mice (105–110, 115, 125). Aldosterone controls expression of Scnn1a, which plays a major role in sodium handling and BP regulation (106–109). The CC gene Per1 participates in the aldosterone-mediated expression of Scnn1a, which further links renal CC machinery and a mediator of sodium balance (126).
Posttranscriptional mechanisms also contribute to the rhythmic gene expression in the kidney (127). Ribosome profiling from mouse kidney revealed a circadian translation pattern for several genes with known roles in renal functions, including aquaporins (Aqp2, Aqp4, Aqp8), podocin (Nphs2), the enzyme Cyp24a1, transporters (Glut9, Pept1), adenosine receptor (Adora1), and Ppara (127). The circadian rhythm of GFR (independent of cardiac function and autonomic nervous system) is critical for maintaining BP. Consistently, podocyte-specific (Nephrin Cre-driven) Bmal1 knockout led to perturbed diurnal GFR and BP (128, 129), raising novel pathophysiological questions about the relationship between podocytes and glomerular hemodynamics. Several renal-derived hormones (angiotensin, endothelins, and aldosterone) display rhythmic production/degradation, and the CC genes Cry1/2 and Per1 are known to participate in this regulation (129–131). Collectively, these investigations confirm that key features of renal physiology (such as BP and electrolyte control and hormone production) display circadian rhythmicity in both humans and mice (132) (Figure 1).
Circadian clock disruption in kidney disease and fibrosis. Chronic kidney disease (CKD) is a global health problem affecting approximately 10% of the population that increases the risks of morbidity and mortality (133–136). CKD may originate from several heterogeneous pathological conditions that damage the cellular structures of kidney, leading to permanent loss of function. The pathogenesis of CKD and renal fibrosis has been extensively reviewed (51, 52, 133, 134). Briefly, sustained pathological insults stemming from multiple conditions (metabolic syndrome/diabetes, drug toxicity, cardiovascular diseases, autoimmunity) lead to immune cell infiltration in the interstitial and glomerular regions (46, 51, 52). This inflammatory response is largely driven through the activation of NF-κB and MAPKs (p38 and JNK), leading to increased production of several pathogenic cytokines, chemokines, and growth factors (46, 51, 52). Persistence of this proinflammatory environment drives activation of pericytes as well as differentiation of myofibroblasts and phenotypic changes characterizing the epithelial-mesenchymal transition, which, along with TGF-β–induced deposition of collagens and fibronectin in the ECM, results in renal fibrosis (46, 51, 52, 133–136). In contrast to liver and lungs, the functional role of the kidney fibroblast–specific CC in health and disease remains unknown (Table 1).
CKD is associated with perturbed diurnal rhythmicity of BP, i.e., a non-dipping pattern, which is independently correlated with a higher mortality rate or predisposition toward end-stage renal disease (105–110, 135). Polymorphisms in the human BMAL1 gene are associated with hypertension and type 2 diabetes (137), both of which predispose to CKD. CKD disrupts sleep patterns (138, 139), which are also documented in animal models of kidney disease (partial nephrectomy, adenine-induced) (105–110). The renin-angiotensin-aldosterone system is altered in CKD, and Bmal1-KO mice display perturbations in renin production, GFR, and BP (140). Thus, excess renin production may contribute to high BP and cardiorenal fibrosis. Another potent profibrotic mediator, the hormone endothelin-1 (ET-1), is a Per1 target gene expressed in collecting duct cells and in the renal cortex (105–108).
Animal models have indicated that CC perturbation predisposes toward renal fibrosis. Notably, genomic-Bmal1-KO mice show aggravated unilateral ureteral obstruction–induced (UUO-induced) interstitial fibrosis (140). Moreover, owing to increased TGF-β activity and Cox2 expression, Clock-KO mice develop more severe renal fibrosis upon ureteral obstruction compared with control mice (141). Whether kidney fibroblast–restricted CC gene mutations aggravate renal fibrosis remains unknown. Deoxycorticosterone acetate–treated (DOCA-treated) mice are a clinically relevant model for developing renal inflammation and fibrosis (142). DOCA alters the renal expression of CC genes, suggesting a link between behavior-induced (diet-induced) alteration of clock and CKD (142). Notably, BMAL1 regulates the circadian expression of Nrf2, a master regulator of antioxidant responses protecting the kidney (143). The NRF2 target genes Hmox1 and Pparg mediate glomerular protection in experimental diabetic glomerulopathy (144) and immune-mediated crescentic glomerulonephritis, respectively (145). Collectively, these investigations suggest that disruption of renal CC function, which normally controls renal physiology, may drive CKD and renal fibrosis. Future studies will be required to unravel the role of kidney cell type–specific (epithelial, fibroblast, and immune) CC mutants in CKD and renal fibrosis (Table 1).
Clock disruptions predispose to chronic respiratory disease and lung fibrosis.
Circadian biology of pulmonary functions. Various aspects of respiratory activity are known to display a 24-hour variation in various species (146–150). Sleep-wake cycle influences the daily rhythmicity of pulmonary ventilation (VE). The metabolic rate drops during sleep and accompanies a decrease in VE. In healthy humans, several aspects of lung function, e.g., forced expiratory volume in 1 second (FEV1), peak expiratory flow (PEF), and airway resistance, display diurnal behavior (146–151). In controlled settings, it was observed that healthy human lungs attain their peak functional capacity (determined by FEV1 and FEV1/FVC [forced vital capacity]) around noon (mid-active phase), which gradually decreases and reaches its lowest level at midnight/early morning (late-rest phase) (151, 152). Recordings of gaseous metabolism and body temperature in rats under controlled conditions have also confirmed the circadian rhythmicity in VE, which closely matches the pattern displayed by the VO2 and VCO2 (measurements of oxygen consumed and carbon dioxide exhaled, respectively, in milliliters per minute). Rhythmicity in VE was also noted in other species, suggesting an evolutionarily conserved phenotype (146). Additionally, in mouse lungs diurnal variations exist for recruitment of immune cells (B cell, granulocytes, and macrophages) (150). These studies indicate that the circadian system contributes to the daily rhythmicity observed for essential pulmonary functions in both humans and mice.
Molecular drivers and successive stages of lung development are considerably similar in mice and humans (153). In utero investigations in mice and rats first detected expression of CC genes at a time frame roughly correlating with the pseudoglandular stage of lung development (E12–E17 in mice, corresponding to 5–17 weeks after conception for humans) (153, 154). A histological study of human and rabbit bronchioles led to the identification of Clara cells (now termed club cells), which play essential roles in maintaining lung homeostasis (153). The transcriptional activity of NKX2.1 (TTF1) is key for lung development and production of surfactant protein A from the Clara cells (153–160). Importantly, TTF1 expression correlates with the transcription of Cry2 and Clock during lung development in mice. In prenatal murine lung, Rev-Erba is presumed to regulate oxidative and inflammatory stress (149). Investigations using Per2-luciferase mice provided the initial evidence for suprachiasmatic nucleus–entrained rhythmic clock in the mature lungs (154). CC genes are expressed in mouse larynx, trachea, bronchus, and lungs (161). Importantly, circadian expression of CC genes and several of the muscarinic acetylcholine receptor genes (Chm2, Chm3, and Chm4) in respiratory tissues of mice was lost following double knockout of Cry1 and Cry2 (Cry1–/–Cry2–/–) (161). Importantly, circadian disruptions modeling shiftwork and jet lag are known to disrupt CC gene expression and lung function (162). Functional CC oscillators are known to be present in different lung cell types, e.g., alveolar and bronchial epithelium (club cells), lung fibroblasts, and macrophages (163–166) (Table 1). Critically, club cells are known to maintain CC coherence among various cell types of lungs, as specific ablation of these cells leads to altered rhythmicity in the remaining tissue (166). Transcriptomic analyses from mouse lungs identified approximately 1,000 genes that are expressed in a diurnal manner, and pathway analyses indicated that the majority are immune related (166). The role of the CC oscillator in pulmonary airway epithelial cells (AECs) was addressed by selective Bmal1 knockout in club cells. RNA sequencing showed that the AEC CC controls expression of genes involved in metabolism of lipids and xenobiotics, ECM remodeling, and chemokine/cytokine signaling (166). Microarray-based study in rats identified numerous genes displaying circadian expression patterns, of which nearly 60% were expressed in the rest (inactive) phase (167). Among the cycling transcripts in rat lungs are genes implicated in maintenance and repair of lung parenchyma, and vasculature (167). Collectively, these investigations have established the “clock” as a major regulator of respiratory functions.
Clock connection to chronic respiratory disease and fibrosis. Chronic respiratory disease is exemplified by chronic obstructive pulmonary disease (COPD) and asthma, which both represent global health concerns affecting quality of life and mortality. Circadian biology influences various aspects of lung disease and fibrosis (53, 54, 146–150, 168). COPD and asthma symptoms worsen in the early morning. A study involving more than 7,000 asthmatic patients reported that around 60% suffered from nighttime symptoms more than three times each week, while 40% suffered every night (168). Multiple factors, including oxidative stress, mucus production/levels, lung inflammation, and cortisol levels, influence the nighttime severity of asthma (168–170). Allergen-driven hyperactivity of eosinophils and mast cells drives bronchospasm. In humans and mice, mast cells and eosinophils are known to harbor functional CCs that regulate the expression of several disease-related genes (171, 172). Notably, IgE-mediated activation of mast cells and consequent release of histamines and leukotrienes were found to exhibit circadian rhythmicity, and Bmal1 and Rev-Erba transcripts were reduced in bronchioles of asthmatic mice (173). In allergic asthma models, macrophage-specific Bmal1-KO mice show increased lung inflammation associated with increased IL-5 levels (173). Furthermore, club cell–specific Bmal1 knockout is known to drive hyperinflammatory responses upon LPS challenge and bacterial infection as a result of perturbed diurnal expression of Cxcl5 (164). Critically, inflammation can disrupt the expression of CC genes in lungs and reprogram the transcriptome and metabolome (146, 147). Collectively, these results suggest that CC perturbation in various cell types of lungs predisposes to inflammatory airway diseases like asthma.
COPD is a chronic disease resulting in reduction of respiratory function. The severity of COPD symptoms in humans shows marked diurnal variation (worsening during late night/early morning) (174, 175). COPD results in oxygen desaturation, thus worsening sleep quality and heightening the risk of mortality due to cardiovascular pathologies (174, 175). A cross-sectional study noted abnormal sleep patterns in approximately 70% of COPD patients, which suggests a feedforward cycle in which sleep disturbance and COPD worsen each other. Chronic exposure to cigarette smoke (CS) is a major cause of COPD (176, 177). In mouse models of COPD, CS altered the expression of Rev-Erba and Per2. Importantly, reduction of REV-ERBα protein levels was observed in COPD patients (146). Genetic studies support a role for REV-ERBα activity in reducing overall inflammation (decreased neutrophil levels and cytokine expression) in COPD (178, 179). Additionally, in mouse lungs, CS-induced COPD reduced levels of Sirt1 (179), a key regulator of CC. Decreases in SIRT1 activity were found in COPD patients (180). Alteration of molecular CC functioning has been linked to pulmonary fibrosis (181–184). Investigations using the ClockΔ19 model showed that the mutants spontaneously develop a fibrotic phenotype with an increase in ECM remodeling gene expression, correlating with higher collagen deposition around bronchioles (181). Mechanistically, CLOCK DNA binding was found to regulate the expression of Nrf2, a regulator of ROS signaling (181). And ClockΔ19 mice presented with reduced Nrf2 and increased oxidative damage (181). REV-ERBα activation could prevent progression of pulmonary fibrosis by limiting TGF-β activity (182, 183). Finally, age-associated lung fibrosis is increased in Bmal1-KO mice (184). Collectively, these investigations reveal that perturbation of the CC is causally linked to chronic respiratory disease and lung fibrosis.
-
Clinical impact: chronotherapeutic basis for targeting diseases
The simultaneous deployment of genetics and various genome-wide approaches in model systems has revealed the widespread role of the clock machinery in maintaining physiology and thus potentially affecting various chronic diseases including fibrosis. Translating these findings to novel therapeutic opportunities will require the integration of different aspects of circadian biology into clinical practice (185–188). Chronopharmacology studies circadian variation to determine optimal timing for drug administration. Indeed, reduced drug toxicity and increased therapeutic efficacy of the colorectal cancer drug oxaliplatin are observed when it is administered in late afternoon versus early morning (189). Time-dependence of treatment response and survival was observed in two recent studies on immunotherapy (190, 191). Moreover, many therapeutic drugs target proteins encoded by genes that are expressed in a circadian manner (192). Thus, chronotherapy holds vast promise for various diseases (185–187).
CC is a major regulator of metabolism. Behavioral modifications, such as restriction of eating to the active phase (in the absence of “binge” eating), improvement of sleep quality, and minimizing of light exposure in rest period, have been shown to improve metabolic homeostasis. Critically, time-restricted eating has been shown to significantly improve obesity, glucose levels, cardiovascular disease, MASH, and liver fibrosis (193–195).
Interestingly, several MASH therapeutic targets are regulated by the CC (4, 5, 23). Indeed, the CC regulates (a) FXR and bile acid metabolism (targeted by FXR agonists), (b) FGF-21 (targeted by antifibrotic compounds in clinical development), and (c) PPAR, the target of elafibranor to treat primary biliary cholangitis. Resmetirom, the first FDA-approved drug for MASH, targets thyroid hormone receptor β to improve mitochondrial fatty acid oxidation and reduce intrahepatic lipid accumulation (196, 197).
In mouse models of metabolic syndrome and MASLD, agonists of CRY (KL001) and REV-ERBs (SR9009 and SR9011) have been shown to improve disease parameters (obesity, glucose tolerance, and lipid profiles) (23, 27). SR9009 reduced expression of profibrotic genes and inflammatory genes, which led to reduction in features of liver fibrosis (92). However, it should be noted that SR9009 displayed REV-ERB–dependent and –independent effects on gene expression. Additionally, REV-ERB activation has been shown to significantly improve lung fibrosis: In different models of lung fibrosis, pharmacological activation of REV-ERB prevented overexpression of collagens and lysyl oxidases (182, 183). Critically, in organotypic cultures from idiopathic pulmonary fibrosis (IPF)patients, REV-ERB agonists prevented the activation of myofibroblasts to IPF-driving fibroblasts that secrete collagen (182, 183). Notably, existing treatments for asthma and COPD (prednisolone and steroid) show circadian variations in their efficacy to reduce inflammation (198). With respect to CKD, multiple observational studies have confirmed that chronotherapeutic methods can improve efficacy of drugs, including furosemide, thiazides, valsartan, and antihypertensive drugs (185–187).
Collectively, the integration of circadian biology into clinical medicine provides novel opportunities to improve the prevention and treatment of chronic diseases and fibrosis. Detailed mechanistic studies as well as the investigation of cell-specific treatment approaches will be required to fully harness CC biology for therapeutic discovery and clinical management.
-
Knowledge gaps and future perspectives
Despite considerable progress in recent years, several knowledge gaps exist (Figure 4 and Table 2). Addressing these gaps will provide opportunities for a better understanding of the impact of CC on disease biology and fibrosis as well as therapeutic discovery. Organs likely crosstalk in a temporally specified (circadian) manner, as this helps to establish synergy between organ-specific gene expression programs and biochemical reactions and to maintain homeostasis. An example of this complexity is the maintenance of glucose homeostasis requiring CC-dictated crosstalk between (a) liver (gluconeogenesis and glycogen synthesis), (b) pancreas (insulin and glucagon production), (c) intestinal release of GLP1 (an approved drug target for obesity), and (d) glucose uptake in skeletal muscle.
 Figure 4
Figure 4Future perspectives and knowledge gaps. In a healthy organ (left), CC-controlled functions maintain homeostasis by driving essential gene expression programs. Emerging evidence suggests that the different CCs also dictate communication between different cell types and organs to maintain physiology. Importantly, perturbation of the CC oscillator function in different cell types alters the rhythmic gene expression programs and drives toward chronic diseases involving fibrosis (right). Although scientific advances have revealed some of the fundamental concepts of circadian biology, several crucial events pertaining to both health and diseased states still remain to be determined (Table 2).
While for some cell types, such as liver and lung epithelial cells or myofibroblasts, common concepts in CC perturbation and disease biology have been described (Table 1), the overarching mechanisms that dictate how various organs crosstalk during chronic disease in a diurnal manner remain largely unknown (Figure 4 and Table 2). The understanding of these mechanisms will provide clues to organ-specific disruption of gene networks. Thus, future investigations should explore at multiple levels (transcriptome, proteome, metabolome) the signals, ligands, and receptors that enable organs to talk to each other, revealing intra- and extracellular maps of communication.
Furthermore, the development of chronic disease and fibrosis in an organ involves interactions between several cell types (epithelial, fibroblasts, and immune cells) with extensive intra- and intercellular crosstalk, influencing disease outcomes. An example is circadian changes in actin polymerization, shown to drive fibroblast mobilization during skin wound healing (199). However, at present the circadian interactions between different cell types in health and disease are largely unknown (Figure 4 and Table 2). Thus, it will be essential to explore, e.g., at the single-cell level, the circadian expression of CC-controlled gene networks that dictate interactions between epithelial, fibroblast, and immune cells.
Additionally, whether polymorphisms in key CC genes, e.g., BMAL1, predispose to chronic diseases and fibrosis of lungs, liver, or other organs remains poorly understood. Furthermore, it will be essential to employ emerging advanced modeling approaches including artificial intelligence and machine learning to integrate data from patients and model organisms to (a) mechanistically understand the common and unique ways of circadian gene regulation in different tissues, and (b) predict the outcome of drug treatments. The understanding of these mechanisms may ultimately lead to discovery of novel targets and drugs.
While there are many mechanistic investigations of circadian biology in mouse models, knowledge from human or patient tissues is rare, which limits the understanding of potential clinical translation. Mice are nocturnal, while human beings are diurnal. Also, murine experimentation is conducted under uniform laboratory conditions (feeding, temperature, humidity, and “light” duration, etc.), which are not observed in day-to-day activity of humans. Furthermore, with respect to CC genes, widely documented variations between individuals confer distinct chronotypes, a term describing human behavioral phenotypes that influence the “timing” of sleep-wake and rest-active periods; these chronotypes are very likely to influence individual disease biology or drug responses. Moreover, how different cell types respond to disease conditions in a circadian manner remains poorly understood. Furthermore, direct targeting of ubiquitously expressed CC genes over the long term may affect multiple organs and have undesirable side effects, which will require additional studies.
Collectively, a detailed investigation of CC oscillator activity and CC-regulated cell circuits in different cell types and organs combined with patient studies for clinical translation will unravel novel approaches to halt progression of chronic disease and prevent or treat fibrosis.
-
Acknowledgments
The authors acknowledge the following financial support: University of Strasbourg Institute of Advanced Science (USIAS) grant 2020-029 (to AM), European Research Council Grant ERC-AdG-2020-FIBCAN #101021417 (to TFB), EU HORIZON-HLTH-2021-DISEASE-04-07 D-SOLVE #101057917 (to TFB), ERC-PoC-2024-FLAMINCO, the French National Research Agency RHU DELIVER (ANR-21-RHUS-0001) (to TFB), LABEX ANR-10-LABX-0028_HEPSYS (to TFB), and the University of Strasbourg Foundation and the Alsace Cancer Foundation (to TFB). This work of the Interdisciplinary Thematic Institute IMCBio, as part of the ITI 2021-2028 program of the University of Strasbourg, CNRS, and Inserm, was further supported by IdEx Unistra (ANR-10-IDEX-0002), and by SFRI-STRAT’US project (ANR 20-SFRI-0012) and EUR IMCBio (ANR-17-EURE-0023) under the framework of the French Investments for the Future Program and the France 2030 program. PLT acknowledges support from Inserm and Fondation pour la Recherche Médicale. DWR is supported by the National Institute for Health and Care Research (NIHR), Oxford Health Biomedical Research Centre, NIHR Oxford Health Biomedical Research Centre grant reference number NIHR203316MRC, MR/W019000/1, and MR/V034049/1. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The authors thank Irene Arrata (Inserm U1110, University of Strasbourg) for graphic layout of the figures.
Address correspondence to: Thomas F. Baumert or Atish Mukherji, Inserm U1110, University of Strasbourg, 3 Rue Koeberlé, 67000 Strasbourg, France. Phone: 33.3.68.85.37.03; Email: thomas.baumert@unistra.fr (TFB); mukherji@unistra.fr (AM).
-
Footnotes
Conflict of interest: Inserm, the University of Strasbourg, the Strasbourg University Hospitals, and the IHU Strasbourg have filed patent applications for the use of anti–claudin-1 monoclonal antibodies for the treatment of fibrosis and cancer (PCT/IB2023/055667, PCT/IB2023/05, 5666PCT/EP2020/081941, PCT/EP2017/056703, PCT/EP2016/055942; TFB, inventor), which have been licensed to Alentis Therapeutics. Additional patent applications on which TFB is an inventor include a method for diagnosis and/or prognosis of liver disease progression and risk of hepatocellular carcinoma (US17896944, US63383441, US63/509362) and discovery of therapeutic compounds and targets to treat liver disease and cancer (PCT/EP2021/072341, PCT/EP2021/055203) as well as a clinical gene signature–based human cell culture model and uses thereof (PCT/EP2016/059477). TFB is founder of, shareholder in, and advisor for Alentis Therapeutics.
Copyright: © 2025, Mukherji et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2025;135(19):e194018. https://doi.org/10.1172/JCI194018.
-
References
- Allada R, Bass J. Circadian mechanisms in medicine. N Engl J Med. 2021;384(6):550–561.
- Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 2019;20(1):49–65.
- Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354(6315):994–999.
- Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93(1):107–135.
- Reinke H, Asher G. Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol. 2019;20(4):227–241.
- Sehgal A. Physiology flies with time. Cell. 2017;171(6):1232–1235.
- Knopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971;68(9):2112–2116.
- Le Gates TA, et al. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;(15):443–454.
- Foster RG, et al. Circadian photoentrainment in mice and humans. Biology (Basel). 2020;9(7):180.
- Ashton A, et al. Photic entrainment of the circadian system. Int J Mol Sci. 2022;23(2):729.
- Mieda M. The central circadian clock of the suprachiasmatic nucleus as an ensemble of multiple oscillatory neurons. Neurosci Res. 2020;156:24–31.
- Dibner C. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549.
- Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164–179.
- Kim YH, Lazar M. Transcriptional control of circadian rhythms and metabolism: a matter of time and space. Endocr Rev. 2020;41(5):707–732.
- Papazyan R, et al. Genetic and epigenomic mechanisms of mammalian circadian transcription. Nat Struct Mol Biol. 2016;23(12):1045–1052.
- Yeung J, et al. Transcription factor activity rhythms and tissue-specific chromatin interactions explain circadian gene expression across organs. Genome Res. 2018;28(2):182–191.
- Beytebiere JR, et al. Tissue-specific BMAL1 cistromes reveal that rhythmic transcription is associated with rhythmic enhancer-enhancer interactions. Genes Dev. 2019;33(5-6):294–309.
- Feng D, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331(6022):1315–1319.
- Mukherji A, et al. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153(4):812–827.
- Solt LA, Burris TP. Action of RORs and their ligands in (patho)physiology. Trends Endocrinol Metab. 2012;23(12):619–627.
- Panda S, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320.
- Storch KF, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417(6884):78–83.
- Mukherji A, et al. The circadian clock and liver function in health and disease. J Hepatol. 2019;71(1):200–211.
- Durrington HJ, et al. The circadian clock and asthma. Thorax. 2014;69(1):90–92.
- Miguel V. Metabolism at the crossroads of inflammation and fibrosis in chronic kidney disease. Nat Rev Nephrol. 2025;21(1):39–56.
- Firsov D, Bonny D. Circadian rhythms and the kidney. Nat Rev Nephrol. 2018;14(10):626–635.
- Mukherji A, et al. Perturbation of the circadian clock and pathogenesis of NAFLD. Metabolism. 2020;111S:154337.
- Weger BD, et al. Systematic analysis of differential rhythmic liver gene expression mediated by the circadian clock and feeding rhythms. Proc Natl Acad Sci U S A. 2021;118(3):e2015803118.
- Vollmers C, et al. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A. 2009;106(50):21453–21458.
- Koronowski KB, Sassone-Corsi P. Communicating clocks shape circadian homeostasis. Science. 2021;371(6530):eabd0951.
- Zhang D, et al. Timing of food intake drives the circadian rhythm of blood pressure. Function (Oxf). 2021;2(1):zqaa034.
- Huang R, et al. Multi-omics profiling reveals rhythmic liver function shaped by meal timing. Nat Commun. 2023;14(1):6086.
- Daniels LJ, et al. Circadian regulation of liver metabolism: experimental approaches in human, rodent, and cellular models. Am J Physiol Cell Physiol. 2023;325(5):1158–1177.
- Bolshette N, et al. Circadian regulation of liver function: from molecular mechanisms to disease pathophysiology. Nat Rev Gastroenterol Hepatol. 2023;20(11):695–707.
- Gachon F, et al. Potential bidirectional communication between the liver and the central circadian clock in MASLD. NPJ Metab Health Dis. 2025;3(1):15.
- Van Gilst D, et al. Effects of the neonatal intensive care environment on circadian health and development of preterm infants. Front Physiol. 2023;14:1243162.
- Escobar C, et al. Development of the circadian system and relevance of periodic signals for neonatal development. Handb Clin Neurol. 2021;179:249–258.
- Cai C, et al. The impact of occupational shift work and working hours during pregnancy on health outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;221(6):563–576.
- Chaves I, et al. Gestational jet lag predisposes to later-life skeletal and cardiac disease. Chronobiol Int. 2019;36(5):657–671.
- Henderson NC, et al. Fibrosis: from mechanisms to medicines. Nature. 2020;587(7835):555–566.
- Talbott HE, et al. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29(8):1161–1180.
- Weicskirchen R, et al. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2019;65:2–15.
- Thannickal VJ, et al. Fibrosis: ultimate and proximate causes. J Clin Invest. 2014;124(11):4673–4677.
- Zhao M, et al. Targeting fibrosis: mechanisms and clinical trials. Signal Transduct Targeted Ther. 2022;7(1):206. View this article via: CrossRef Google Scholar
- Vos T, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222.
- Rockey DC, et al. Fibrosis—a common pathway to organ injury and failure. N Engl J Med. 2015;372(12):1138–1149.
- Majo J, et al. Pathology and natural history of organ fibrosis. Curr Opin Pharmacol. 2019;49:82–89.
- Roehlen N, et al. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. 2020;9(4):875.
- Friedman SL, Pinzani M. Hepatic fibrosis 2022: unmet needs and a blueprint for the future. Hepatology. 2022;75(2):473–488.
- Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18(3):151–166.
- Humphreys BD. Mechanisms of renal fibrosis. Annu Rev Physiol. 2018;80:309–326.
- Panizo S, et al. Fibrosis in chronic kidney disease: pathogenesis and consequences. Int J Mol Sci. 2021;22(1):408.
- Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811–1823.
- Koudstaal T, et al. Pulmonary fibrosis: from pathogenesis to clinical decision-making. Trends Mol Med. 2023;29(12):1076–1087.
- Garcia CK. Insights from human genetic studies of lung and organ fibrosis. J Clin Invest. 2018;128(1):36–44.
- Nieto MA, et al. EMT 2016. Cell. 2016;166(1):21–45.
- Hammerich L, Tacke F. Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol. 2023;20(10):633–646.
- Peiseler M. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease — novel insights into cellular communication circuits. J Hepatol. 2022;77(4):1136–1160.
- Mack M. Inflammation and fibrosis. Matrix Biol. 2018;68-69:106–121.
- Massagué J, Sheppard D. TGF-β signaling in health and disease. Cell. 2023;186(19):4007–4037.
- Kim KK, et al. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol. 2018;10(4):a022293.
- David CJ, Massagué J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat Rev Mol Cell Biol. 2018;19(7):419–435.
- Lathan R. Exploring unconventional targets in myofibroblast transdifferentiation outside classical TGF-β signaling in renal fibrosis. Front Physiol. 2024;15:1296504.
- Koike N, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349–354.
- Rey G, et al. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 2011;9(2):e1000595.
- Fang B, et al. Circadian enhancers coordinate multiple phases of rhythmic gene transcription in vivo. Cell. 2014;159(5):1140–1152.
- Menet JS, et al. CLOCK:BMAL1 is a pioneer-like transcription factor. Genes Dev. 2014;28(1):8–13.
- Sato S, et al. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell. 2017;170(4):664–677.
- Le Martelot G, et al. Genome-wide RNA polymerase II profiles and RNA accumulation reveal kinetics of transcription and associated epigenetic changes during diurnal cycles. PLoS Biol. 2012;10(11):e1001442.
- Mermet J, et al. Clock-dependent chromatin topology modulates circadian transcription and behavior. Genes Dev. 2018;32(5-6):347–358.
- Kim YH, et al. Rev-erbα dynamically modulates chromatin looping to control circadian gene transcription. Science. 2018;359(6381):1274–1277.
- Furlan-Magaril M, et al. The global and promoter-centric 3D genome organization temporally resolved during a circadian cycle. Genome Biol. 2021;22(1):162.
- Paris J, Henderson NC. Liver zonation, revisited. Hepatology. 2022;76(4):1219–1230.
- Droin C, et al. Space-time logic of liver gene expression at sub-lobular scale. Nat Metab. 2021;3(1):43–58.
- Guan D, et al. The hepatocyte clock and feeding control chronophysiology of multiple liver cell types. Science. 2020;369(6509):1388–1394.
- Sugimoto A, et al. Hepatic stellate cells control liver zonation, size and functions via R-spondin 3. Nature. 2025;640(8059):752–761.
- Robles MS, et al. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet. 2014;10(1):e1004047.
- Mauvoisin D, et al. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc Natl Acad Sci U S A. 2014;111(1):167–172.
- Wang J, et al. Nuclear proteomics uncovers diurnal regulatory landscapes in mouse liver. Cell Metab. 2017;25(1):102–117.
- Aviram R, et al. Lipidomics analyses reveal temporal and spatial lipid organization and uncover daily oscillations in intracellular organelles. Mol Cell. 2016;62(4):636–648.
- Adamovich Y, et al. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014;19(2):319–330.
- Panda S. Circadian physiology of metabolism. Science. 2016;354(6315):1008–1015.
- Liu C, et al. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447(7143):477–481.
- Lamia KA, et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480(7378):552–556.
- Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–1045.
- Solt LA, et al. Regulation of circadian behavior and metabolism by synthetic REV-ERB agonists. Nature. 2012;485(7396):62–68.
- Bugge A, et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26(7):657–667.
- Le Martelot G, et al. REV-ERBα participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7(9):e1000181.
- Chaix A, et al. The circadian coordination of cell biology. J Cell Biol. 2016;215(1):15–25.
- Jacobi D, et al. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab. 2015;22(4):709–720.
- Schrader LA, et al. Circadian disruption, clock genes, and metabolic health. J Clin Invest. 2024;134(14):e170998.
- Crouchet E, et al. Targeting the liver clock improves fibrosis by restoring TGF-β signaling. J Hepatol. 2025;82(1):120–133.
- Delbes AS, et al. Mice with humanized livers reveal the role of hepatocyte clocks in rhythmic behavior. Sci Adv. 2023;9(20):eadf2982.
- Mukherji A, Jühling F, et alAn atlas of the human liver diurnal transcriptome and its perturbation by hepatitis C virus infection. Nat Commun. 2024;15(1):7486.
- Grompe M. Mice with human livers. Gastroenterology. 2013;145(6):1209–1214.
- Juhling F. Targeting clinical epigenetic reprogramming for chemoprevention of metabolic and viral hepatocellular carcinoma. Gut. 2021;70(1):157–169.
- Rinella ME, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–1556.
- Sanyal AJ, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385(17):1559–1569.
- Hagstrom P, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–1273.
- Mota M, et al. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65(8):1049–1061.
- Vacca M, et al. An unbiased ranking of murine dietary models based on their proximity to human metabolic dysfunction-associated steatotic liver disease (MASLD). Nat Metab. 2024;6(6):1178–1196.
- Mukherji A, et al. Shifting eating to the circadian rest phase, misaligns the peripheral circadian clocks with the master SCN clock, which leads to a metabolic syndrome. Proc Natl Acad Sci U S A. 2015;112(48):6691–6698.
- Zheng R, et al. Chronic jet lag alters gut microbiome and mycobiome and promotes the progression of MAFLD in HFHFD-fed mice. Front Microbiol. 2023;14:1295869.
- Meng Q, et al. Circadian regulator BMAL1:CLOCK promotes cell proliferation in hepatocellular carcinoma by controlling apoptosis and cell cycle. Proc Natl Acad Sci U S A. 2023;120(2):e2214829120.
- Solocinski K, Gumz ML. The circadian clock in the regulation of renal rhythms. J Biol Rhythms. 2015;30(6):470–486.
- Crislip GR, et al. Recent advances in understanding the circadian clock in renal physiology. Curr Opin Physiol. 2018;5:38–44.
- Mohandas R, et al. Circadian rhythms and renal pathophysiology. J Clin Invest. 2022;132(3):e148277.
- Costello HM, et al. Circadian clocks of the kidney: function, mechanism, and regulation. Physiol Rev. 2022;102(4):1669–1701.
- Bonny O, Firsov D. Circadian regulation of renal function and potential role in hypertension. Curr Opin Nephrol Hypertens. 2013;22(4):439–444.
- Shea SA, et al. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108(8):980–984.
- Armstrong JA. Urinalysis in Western culture: a brief history. Kidney Int. 2007;71(5):384–387.
- Moore-Ede MC, Herd JA. Renal electrolyte circadian rhythms: independence from feeding and activity patterns. Am J Physiol. 1977;232(2):F128–F135.
- Frommer JP, Ayus JC. Effect of minor surgery on urinary flow and electrolyte excretion in the awake dog. Miner Electrolyte Metab. 1985;11(2):91–96.View this article via: PubMed Google Scholar
- Pons M, et al. Circadian rhythms in renal function in hypertensive TGR(mRen-2)27 rats and their normotensive controls. Am J Physiol. 1996;271(4 pt 2):1002–1008.
- Zuber AM, et al. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A. 2009;106(38):16523–16528.
- Nikolaeva S, et al. The circadian clock modulates renal sodium handling. J Am Soc Nephrol. 2012;23(6):1019–1026.
- Mills JN. Diurnal rhythm in urine flow. J Physiol. 1951;113(4):528–536.
- Mills JN, Stanbury SW. Intrinsic diurnal rhythm in urinary electrolyte output. J Physiol. 1951;115(1):18p–19p.View this article via: PubMed Google Scholar
- Mills JN. Circadian rhythms during and after three months in solitude underground. J Physiol. 1964;174(2):217–231.
- Johnston JG, et al. Diurnal control of blood pressure is uncoupled from sodium excretion. Hypertension. 2020;75(6):1624–1634.
- Crislip GR, et al. Differences in renal BMAL1 contribution to Na+ homeostasis and blood pressure control in male and female mice. Am J Physiol Renal Physiol. 2020;318(6):1463–1477.
- Dolatshad H, et al. Differential expression of the circadian clock in maternal and embryonic tissues of mice. PLoS One. 2010;5(3):e9855.
- Dan H, et al. Circadian clock regulation of developmental time in the kidney. Cell Rep. 2020;31(7):107661.
- Mészáros K, et al. Development of the circadian clockwork in the kidney. Kidney Int. 2014;86(5):915–922.
- Pradervand S, et al. A comprehensive analysis of gene expression profiles in distal parts of the mouse renal tubule. Pflugers Arch. 2010;460(6):925–952.
- Gumz ML, et al. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest. 2009;119(8):2423–2434.
- Castelo-Szekely V, et al. Translational contributions to tissue specificity in rhythmic and constitutive gene expression. Genome Biol. 2017;18(1):116.
- Gumz ML. Taking into account circadian rhythm when conducting experiments on animals. Am J Physiol Renal Physiol. 2016;310(6):F454–F455.
- Ansermet C, et al. The intrinsic circadian clock in podocytes controls glomerular filtration rate. Sci Rep. 2019;9(1):16089.
- Doi M, et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16(1):67–74.
- Douma LG, et al. Kidney-specific KO of the circadian clock protein PER1 alters renal Na+ handling, aldosterone levels, and kidney/adrenal gene expression. Am J Physiol Renal Physiol. 2022;322(4):F449–F459.
- Juffre A, Gumz ML. Recent advances in understanding the kidney circadian clock mechanism. Am J Physiol Renal Physiol. 2024;326(3):382–393.
- Romagnani P, et al. Chronic kidney disease. Nat Rev Dis Primers. 2017;3:17088.
- Yamashita N, Kramann R. Mechanisms of kidney fibrosis and routes towards therapy. Trends Endocrinol Metab. 2024;35(1):31–48.
- Webster AC, et al. Chronic kidney disease. Lancet. 2017;389(10075):1238–1252.
- Kuppe C, et al. Decoding myofibroblast origins in human kidney fibrosis. Nature. 2021;589(7841):281–286.
- Woon PY, et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104(36):14412–14417.
- Patke A, et al. Mutation of the human circadian clock gene CRY1 in familial delayed phase sleep disorder. Cell. 2017;169(2):203–215.
- Dashti HS et al. Clock genes explain a large proportion of phenotypic variance in systolic blood pressure and this control is not modified by environmental temperature. Am J Hypertens. 2016;29(1):132–140.
- Zhang J, et al. Postnatal deletion of Bmal1 in mice protects against obstructing renal fibrosis via suppressing Gli2 transcription. FASEB J. 2021;35(5):e21530.
- Chen WD, et al. Circadian CLOCK mediates activation of transforming growth factor-β signaling and renal fibrosis through cyclooxygenase 2. Am J Pathol. 2015;185(12):3152–3163.
- Fletcher EK, et al. Deoxycorticosterone/salt-mediated cardiac inflammation and fibrosis are dependent on functional CLOCK signaling in male mice. Endocrinology. 2017;158(9):2906–2917.
- Sun Q et al. Mechanism of circadian regulation of the NRF2/ARE pathway in renal ischemia-reperfusion. Exp Ther Med. 2021;21(3):190.
- Lenoir O, et al. Hmox1 deficiency sensitizes mice to peroxynitrite formation and diabetic glomerular microvascular injuries. J Diabetes Res. 2017;2017:9603924.
- Henique C, et al. Nuclear factor erythroid 2-related factor 2 drives podocyte-specific expression of peroxisome proliferator-activated receptor γ essential for resistance to crescentic GN. J Am Soc Nephrol. 2016;27(1):172–188.
- Ince LM, et al. The lung mast cell: its physiology and potential relevance to defense of the lung. Environ Health Perspect. 2018;35:153–164.
- Cunningham PS, et al. Circadian regulation of pulmonary disease: the importance of timing. Clin Sci (Lond). 2023;137(11):895–912.
- Spenglar CM, Shea S. Endogenous circadian rhythm of pulmonary function in healthy humans. Am J Respir Crit Care Med. 2000;162(3 pt 1):1038–1046.
- Bartman CM, Prakash YS. Bringing the cellular clock into understanding lung disease: it’s time, period! Am J Physiol Lung Cell Mol Physiol. 2020;319(2):273–276.
- Gibbs J. Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology. 2009;150(1):268–276.
- Medarov BL, et al. Diurnal variations in human pulmonary function. Int J Clin Exp Med. 2008;1(3):267–273.View this article via: PubMed Google Scholar
- Barnes PJ. Circadian variation in airway function. Am J Med. 1985;79(6a):5–9.
- Bartman C. It’s about time: clocks in the developing lung. J Clin Invest. 2020;130(1):39–50.
- Yoo SH, et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101(15):5339–5346.
- O’Neill JS, Reddy AB. The essential role of cAMP/Ca2+ signalling in mammalian circadian timekeeping. Biochem Soc Trans. 2012;40(1):44–50.
- Gonzales LW, et al. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):940–951.
- Boggaram V. Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clin Sci (Lond). 2009;116(1):27–35.
- Tagne JB, et al. Genome-wide analyses of Nkx2-1 binding to transcriptional target genes uncover novel regulatory patterns conserved in lung development and tumors. PLoS One. 2012;7(1):e29907.
- Wu Y, et al. Reciprocal regulation between the circadian clock and hypoxia signaling at the genome level in mammals. Cell Metab. 2017;25(1):73–85.
- Chen L, Yang G. PPARs integrate the mammalian clock and energy metabolism. PPAR Res. 2014;2014:653017.
- Bando H, et al. Vagal regulation of respiratory clocks in mice. J Neurosci. 2007;27(16):4359–4365.
- Hadden H, et al. Circadian disruption alters mouse lung clock gene expression and lung mechanics. J Appl Physiol (1985). 2012;113(3):385–392.
- Meng QJ, et al. Ligand modulation of REV-ERBalpha function resets the peripheral circadian clock in a phasic manner. J Cell Sci. 2008;121(pt 21):3629–3635.
- Gibbs JE, et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med. 2014;20(8):919–926.
- Putker M, et al. Mammalian circadian period, but not phase and amplitude, is robust against redox and metabolic perturbations. Antioxid Redox Signal. 2018;28(7):507–520.
- Zhang Z, et al. Genome-wide effect of pulmonary airway epithelial cell-specific Bmal1 deletion. FASEB J. 2019;33(5):6226–6238.
- Sukumaran S, et al. Light-dark oscillations in the lung transcriptome: implications for lung homeostasis, repair, metabolism, disease, and drug action. J Appl Physiol (1985). 2011;110(6):1732–1747.
- Litinski M, et al. Influence of the circadian system on disease severity. Sleep Med Clin. 2009;4(2):143–163.
- Kelley EA, et al. Inflammatory changes associated with circadian variation in pulmonary function in subjects with mild asthma. Clin Exp Allergy. 2004;34(2):227–233.
- Hetzel MR, Clark TJ. Comparison of normal and asthmatic circadian rhythms in peak expiratory flow rate. Thorax. 1980;35(10):732–738.
- Baumann A, et al. The circadian clock is functional in eosinophils and mast cells. Immunology. 2013;140(4):465–474.
- Nussbaum JC, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–248.
- Zaslona Z, et al. The circadian protein BMAL1 in myeloid cells is a negative regulator of allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2017;312(6):L855–L860.
- Garrow AP, et al. The development and first validation of the Manchester Early Morning Symptoms Index (MEMSI) for patients with COPD. Thorax. 2015;70(8):757–763.
- Ding B, et al. A cross-sectional survey of night-time symptoms and impact of sleep disturbance on symptoms and health status in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:589–599.
- Vasu VT, et al. Nr1d1, an important circadian pathway regulatory gene, is suppressed by cigarette smoke in murine lungs. Integr Cancer Ther. 2009;8(4):321–328.
- Lechasseur A, et al. Exposure to electronic cigarette vapors affects pulmonary and systemic expression of circadian molecular clock genes. Physiol Rep. 2017;5(19):e13440.
- Pariollaud M, et al. Circadian clock component REV-ERBα controls homeostatic regulation of pulmonary inflammation. J Clin Invest. 2018;128(6):2281–2296.
- Sundar IK, et al. The nuclear receptor and clock gene REV-ERBα regulates cigarette smoke-induced lung inflammation. Biochem Biophys Res Commun. 2017;493(4):1390–1395.
- Yao H, et al. Disruption of sirtuin 1-mediated control of circadian molecular clock and inflammation in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2015;53(6):782–792.
- Pekovic-Vaughan V, et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014;28(6):548–560.
- Cunningham PS, et al. The circadian clock protein REVERBα inhibits pulmonary fibrosis development. Proc Natl Acad Sci U S A. 2020;117(2):1139–1147.
- Wang Q, et al. Circadian clock molecule REV-ERBα regulates lung fibrotic progression through collagen stabilization. Nat Commun. 2023;14(1):1295.
- Sundar IK, et al. Influenza A virus-dependent remodeling of pulmonary clock function in a mouse model of COPD. Sci Rep. 2015;4:9927.
- Ballesta A, et al. Systems chronotherapeutics. Pharmacol Rev. 2017;69(2):161–199.
- Dallmann R, et al. Chronopharmacology: new insights and therapeutic implications. Annu Rev Pharmacol Toxicol. 2014;54:339–361.
- Cederroth CR, et al. Medicine in the fourth dimension. Cell Metab. 2019;30(2):238–250.
- Lévi F, et al. Circadian regulation of drug responses: toward sex-specific and personalized chronotherapy. Annu Rev Pharmacol Toxicol. 2024;64:89–114.
- Caussanel JP, et al. Phase I trial of 5-day continuous venous infusion of oxaliplatin at circadian rhythm-modulated rate compared with constant rate. J Natl Cancer Inst. 1990;82(12):1046–1050.
- Barrios CH, et al. Time-of-day infusion of immunotherapy may impact outcomes in advanced non-small cell lung cancer patients (NSCLC). J Clin Oncol. 2022;40(suppl 16):e21126. View this article via: CrossRef Google Scholar
- Zhang Y, et al. Randomized trial of relevance of time-of-day of immunochemotherapy for progression-free and overall survival in patients with non-small cell lung cancer. J Clin Oncol. 2025;43(suppl 16):8516. View this article via: CrossRef Google Scholar
- Zhang R, et al. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111(45):16219–16224.
- Lagarde D, Kazak L. The timing of eating controls energy use. Science. 2022;378(6617):251–252.
- Gallage S, et al. A 5:2 intermittent fasting regimen ameliorates NASH and fibrosis and blunts HCC development via hepatic PPARα and PCK1. Cell Metab. 2024;36(6):1371–1393.
- Chaix A, et al. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 2019;29(2):303–319.
- Harrison SA, et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390(6):497–509.
- Francque S, et al. A turning point in hepatology? EASL reflects on the first approved drug for MASH. J Hepatol. 2024;81(2):192–194.
- Giri A, et al. Circadian clock-based therapeutics in chronic pulmonary diseases. Trends Pharmacol Sci. 2022;43(12):1014–1029.
- Hoyle NP, et al. Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci Transl Med. 2017;9(415):eaal2774.
-
Version history
- Version 1 (October 1, 2025): Electronic publication
Article tools
-
View PDF

- Download citation information
- Send a comment
- Terms of use
- Standard abbreviations
- Need help? Email the journal
Metrics
Go to
- Top
- Abstract
- Introduction to the circadian clock: principles and components
- Fibrosis: underlying common principles and molecular drivers
- Circadian drivers of metabolic liver disease and fibrosis
- Circadian disruption in chronic kidney disease and fibrosis
- Clinical impact: chronotherapeutic basis for targeting diseases
- Knowledge gaps and future perspectives
- Acknowledgments
- Footnotes
- References
- Version history



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)






