Advertisement
Review Series
Open Access |  10.1172/JCI188348
10.1172/JCI188348
The complement system in intestinal inflammation and cancer
Carsten Krieg1 and Silvia Guglietta2,3
1Department of Pathology and Laboratory Medicine and
2Department of Regenerative Medicine & Cell Biology, Medical University of South Carolina, Charleston, South Carolina, USA.
3Hollings Cancer Center, Charleston, South Carolina, USA.
Address correspondence to: Silvia Guglietta, Hollings Cancer Center, Medical University of South Carolina, Department of Regenerative Medicine & Cell Biology, 173 Ashley Ave., CRI609, Charleston, South Carolina 29425-8908, USA. Phone: 843.792.9702; Email: gugliett@musc.edu.
Find articles by
Krieg, C.
in:
PubMed
|
Google Scholar
|

1Department of Pathology and Laboratory Medicine and
2Department of Regenerative Medicine & Cell Biology, Medical University of South Carolina, Charleston, South Carolina, USA.
3Hollings Cancer Center, Charleston, South Carolina, USA.
Address correspondence to: Silvia Guglietta, Hollings Cancer Center, Medical University of South Carolina, Department of Regenerative Medicine & Cell Biology, 173 Ashley Ave., CRI609, Charleston, South Carolina 29425-8908, USA. Phone: 843.792.9702; Email: gugliett@musc.edu.
Find articles by
Guglietta, S.
in:
PubMed
|
Google Scholar
|

Published October 1, 2025 - More info
J Clin Invest. 2025;135(19):e188348. https://doi.org/10.1172/JCI188348.
© 2025 Krieg et al. This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
-
Abstract
The complement system has emerged as a critical regulator of intestinal homeostasis, inflammation, and cancer. In this Review, we explore the multifaceted roles of complement in the gastrointestinal tract, highlighting its canonical and noncanonical functions across intestinal epithelial and immune cells. Under homeostatic conditions, intestinal cells produce complement that maintains barrier integrity and modulates local immune responses, but complement dysregulation contributes to intestinal inflammation and promotes colon cancer. We discuss recent clinical and preclinical studies to provide a cohesive overview of how complement-mediated modulation of immune and nonimmune cell functions can protect or exacerbate inflammation and colon cancer development. The complement system plays a dual role in the intestine, with certain components supporting tissue protection and repair and others exacerbating inflammation. Intriguingly, distinct complement pathways modulate colon cancer progression and response to therapy, with novel findings suggesting that the C3a/C3aR axis constrains early tumor development but may limit antitumor immunity. The recent discovery of intracellular complement activation and tissue-specific complement remains vastly underexplored in the context of intestinal inflammation and colon cancer. Collectively, complement functions are context- and cell-type-dependent, acting both as a shield and a sword in intestinal diseases. Future studies dissecting the temporal and spatial dynamics of complement are essential for leveraging its potential as a biomarker and therapeutic in colon cancer.
-
Introduction
The intestinal tract is a highly specialized organ system responsible not only for nutrient absorption and waste excretion, but also for maintaining a delicate balance between host immunity and tolerance to the vast array of microbial and environmental stimuli it encounters. Central to this equilibrium is the integrity of the intestinal barrier, which relies on tightly regulated epithelial, immune, and microbial interactions. The complement system — traditionally known for its role in pathogen defense — has recently gained recognition for its critical functions in maintaining intestinal homeostasis and mediating responses to injury and infection. Beyond its classical extracellular roles, complement proteins are now understood to act locally and intracellularly, influencing a wide spectrum of physiological and pathological processes. This Review explores how dysregulation of the complement system contributes to intestinal inflammation and cancer, with a focus on emerging mechanisms, cell-specific functions, and potential therapeutic implications.
-
Intestinal compartmentalization
The intestine is equipped with a multilayer and multifunctional barrier composed by mucus, specialized intestinal epithelial cells (IECs) that provide a physical and chemical barrier, and immune cells (Figure 1). Alterations of virtually any of the single components of the intestinal barrier can result in the disruption of intestinal homeostasis and development of inflammation. Enhanced permeability promotes the translocation of luminal microbes across the barrier, ultimately initiating an inflammatory immune response involving production of inflammatory cytokines that further contribute to the disruption of the epithelial barrier and exacerbate intestinal inflammation, as extensively reviewed by Horowitz and collaborators (1). Moreover, as we discuss below, components of the complement system influence intestinal barrier integrity in complex ways. Breakdown of the intestinal barrier and subsequent loss of tolerance creates a cycle of inflammation that contributes to the pathogenesis of intestinal diseases such as inflammatory bowel disease (IBD), in which altered expression of various tight junction proteins has been observed (2–4).
 Figure 1
Figure 1The composition of the intestinal barrier. The intestinal mucus creates a physical and chemical barrier that prevents direct contact between the luminal microbiota and the intestinal epithelium. Along the small intestine (SI), the mucus layer is relatively porous to different bacteria and thin. The colon (C) is equipped with a double mucus layer: the outer layer is relatively penetrable to bacteria; the inner layer is highly impenetrable to the microbiome (120–123). The intestinal epithelium comprises a single layer of heterogenous and highly specialized cell subtypes arising from common stem cell progenitors (Lgr5+ cells) at the bottom of the crypts. Intestinal epithelial cells (IECs) are tightly held together via strong cell adhesion proteins, forming a physical barrier that separates the contents of the intestinal lumen from the underlying lamina propria (124). Along with providing a physical barrier, cells within the intestinal epithelium produce and secrete molecules that help maintain intestinal homeostasis via a chemical barrier (124). Enterocytes (SI) and colonocytes (C) are the most abundant IECs. These cells recognize pathogen-associated molecular patterns via TLRs and NLRs (125–128). Enterocytes also express polymeric IgA receptors (pIgR) basolaterally, limiting intestinal permeability and susceptibility to intestinal inflammation and infections. Additional IEC types are goblet cells, which are responsible for the formation of the mucus layer through secretion of Muc2 mucin as well as other proteins; Paneth cells, which are found in the base of crypts within the SI that secrete antimicrobial peptides (AMP); tuft cells, which provide defense against helminth infections; and enteroendocrine cells, which secrete hormones. Intraepithelial lymphocytes (IELs) such as CD8+ T and γδ T cells are also found in the intestinal epithelium. Most immune cells are located in the lamina propria underneath the epithelial layer and in the gut-associated lymphoid tissues, which represent the key antigen sampling and adaptive immune inductive sites within the intestinal wall.
-
The complement system
The complement system represents one of the most ancient and conserved mechanisms of defense, which has been transmitted throughout the evolution from invertebrates to humans (5). Besides playing a key role in the defense against infections, an overwhelming number of studies have shown that the complement system is critical in maintaining health and homeostasis in the entire human body and that its functions can go well beyond providing defense against pathogens. As such, it is not surprising that dysregulation of the complement system has been described in the development and progression of diseases spanning broad etiologies and arising in multiple body sites, from autoimmunity to neuroinflammation and cancer, as reviewed elsewhere (6–9). The complement system comprises more than 50 proteins with major roles in the innate immune response against pathogens and altered host cells via opsonization, inflammation, and cell lysis. These proteins can be found in circulation, on the cell surface, or within cells and play critical roles in the initiation, amplification, effector function, or regulation of the complement cascade. In the complement cascade, a series of enzymatic cleavages are initiated upon the recognition of foreign or altered cells by various complement proteins (10). The cascade can be initiated via the classical, the lectin, or the alternative pathways. Although distinct, all three pathways lead to the generation of the C3 convertases, which mediate the cleavage of C3 into C3a and C3b and allow the formation of the C5 convertase. This step is essential to produce the downstream proteins that form the membrane attack complex (MAC) (11). To prevent aberrant activation, the complement system possesses distinct regulatory mechanisms that target specific steps within the cascade (12–14) (Figure 2).
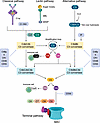 Figure 2
Figure 2Complement activation and regulation. Binding of C1q to IgG or IgM antibody–opsonized pathogens or altered host cells initiates the classical pathway, whereas binding of MBL to sugar motifs on the surface of bacteria initiates the lectin pathway. The alternative pathway is initiated via spontaneous cleavage of the complement protein C3 into C3a and C3b. All three pathways converge onto the complement protein C3, which is cleaved into C3a and C3b by the C3 convertases (C4b2a and C3bCBb). The generation of C3 convertases amplifies the complement cascade through continued cleavage of C3. C3b opsonizes microbes and targets them for destruction by phagocytes. C3b combines with other complement proteins to generate the C5 convertases (C4b2a3b and C3bBb3b), which cleave C5 into C5a and C5b (14, 129). C3a and C5a are complement anaphylatoxins that bind to their respective cell surface receptors, C3aR and C5aR, and promote migration to and activation of immune cells at sites of infection or damage (130, 131). C5b binds to complement proteins C6, C7, C8, and C9 to generate the MAC, which forms a pore in the cell membrane and induces lysis of bacterial or complement-targeted host cells (11). CD55 is a membrane-bound complement regulator that disassembles C3 and C5 convertases to prevent amplification of the cascade and the generation of the MAC (12). CD46, also known as membrane cofactor protein (MCP), works with serum factor I to prevent C3 convertase reassembly and amplification of the cascade. CD46 functions are well characterized in humans but a functional mouse homolog has not yet been identified (132). CD59 is a complement regulator that blocks MAC formation. The complement regulator CR1, which exists in a soluble (sCR1) and membrane-bound form, acts as a receptor for C3b and C4b, thereby destabilizing and enhancing decay of the classical and alternative pathway C3 and C5 convertases (133).
Traditionally, cleavage of C3 into its soluble and membrane-bound fragments was believed to be confined to the extracellular space both for systemic and cell-derived complement. However, seminal work by Kemper and collaborators revealed the existence of an intracellular complement system, termed the complosome, whereby the production and activation of the cascade remain confined within cells (15). These studies shed light on novel noncanonical functions of the complement system, sparking exciting research that is fundamentally changing our understanding of complement and its roles in health and diseases. Besides multiple immune cell types, cell-autonomous complement has been described in endothelial and epithelial cells in intestine, lung, bladder, kidney, and eye as well as in pancreatic β cells, hepatocytes, fibroblasts, and neurons, orchestrating responses to infectious and noninfectious stimuli (16–21). More extensive reviews on the functions of intracellular complement have been previously published (6, 8, 22). However, evidence for a role of the intracellular complement in intestinal inflammation and colon cancer is still limited, so here we focus primarily on extracellular complement signaling.
As an essential component of host immunity, the complement system is gaining increasing attention in the gastrointestinal (GI) tract, where its functions have been described in intestinal homeostasis, inflammation, and cancer. In this context, a recent study by Wu and collaborators provided compelling evidence for the existence of a gut-intrinsic complement system that acts independently from liver-derived complement, further validating the concept of tissue-specific complement already described in lung and brain (23). In the intestine, the complement system cooperates with other innate sensing mechanisms. Multiple studies have provided evidence for crosstalk between the complement system and the TLRs (24–26). More recently, Kopp et al. speculated that there may be crosstalk between complement and Paneth cells, whereby complement proteins such as the anaphylatoxins C3a and C5a and/or the MAC could enhance or dampen antimicrobial peptide (AMP) production or whereby AMPs drive the intracellular production of C3 and C5 by specialized IECs (27). This hypothesis is supported by a study by Cheoub et al. showing that C5aR antagonism resulted in a decline in diversity of the skin microbiota of healthy mice associated with changes in immune effectors, including AMPs. Besides its crosstalk with other innate sensing mechanisms, multiple components of the complement system regulate the function of IECs and immune cells, thus contributing to intestinal homeostasis in the healthy intestine (Figure 3A).
 Figure 3
Figure 3Complement in healthy colon, IBD, and colon cancer. (A) In healthy colon local C3 production is dependent on the commensal flora, and stromal and myeloid cells are the major producers (23). IECs constitutively produce and secrete C3 and express the C3aR and TLR1–4. C3a and its degradation product C3a-DesArg have potent antibacterial and antifungal properties independent of their interaction with C3aR (134). Primary IECs do not express C5aR, indicating that C5aR expression may be more prominent during intestinal inflammation (135, 136). Lgr5+ stem cells in the SI express C3aR, which acts as an autocrine growth factor necessary for the proliferation of the intestinal organoids (137). C3aR is mostly expressed in lamina propria eosinophils, CD11b+ and CD103+ conventional dendritic cells, plasmacytoid dendritic cells, and a fraction of innate lymphoid cells (138). Tregs, macrophages, and dendritic cells support intestinal homeostasis. (B) In inflammatory bowel disease, the reduced mucus layer enables proliferation of pathobionts and their proximity to the intestinal epithelium, causing migration of innate and adaptive immune cells, inflammation and disruption of the intestinal barrier. C3 activation leads to opsonization of mucosa-associated bacteria by C3b. Crohn’s disease involves Th1/Th17 inflammation and higher levels of C3, C1q, and CfB. Ulcerative colitis results in Th2 inflammation and no induction of C3. (C) Alterations of the mucus layers and microbiota occur in colon cancer. Untreated tumors express CD55 and C5aR. Inflammasome activation results in cleavage of C1qbp, preventing its translocation to the mitochondria, inhibiting OXPHOS, and supporting cancer cell proliferation. C5aR-expressing neutrophils produce IL-1β, which stimulates macrophage IL-17 production. C3aR on neutrophils induces NETosis. Both mechanisms support tumor growth. Radiation therapy induces C5aR overexpression on cancer cells. Treatment with a C5aR antagonist causes tumor cell death and improved response to radiotherapy. Loss of C3aR results in increased recruitment of Th1, Th17, and cytotoxic CD8 cells and improved response to anti–PD-1 therapy.
-
Role of the complement system in intestinal inflammation
As the largest mucosal surface of the human body, the GI tract is constantly exposed to environmental stimuli, mainly via the introduction of new antigens derived from food, toxins, and the luminal flora. The highly compartmentalized structure of the intestine allows selective absorption of nutrients and water while selectively limiting permeation of luminal toxins, antigens, and pathobionts through the mucosa, ultimately preventing excessive inflammation. Disruption of this delicate balance can result in intestinal inflammation and in the development of IBD, which has been the subject of multiple investigations in the complement field. Crohn’s disease (CD) and ulcerative colitis (UC) are the two main forms of IBD, and they differ in terms of location, histopathology, and immune responses, as extensively reviewed by others (28, 29). Since the complement system plays key roles in innate defense and in the regulation of various cell functions, it is not surprising that many investigations have focused on studying its relevance in the context of IBD. These studies are summarized in Table 1. An overview of the involvement of complement components in IBD is provided in Figure 3B.
Complement system in patients with IBD. The first evidence for complement involvement in IBD came from studies showing increased levels of complement factor B (CfB) and C3 in the sera of patients with IBD and their correlation with disease activity (30, 31). Additional evidence of complement activation at the protein level in patients with IBD came from a study by Ueki and collaborators, which investigated the expression of C3 activation and degradation products, terminal complement complex (TCC), and complement regulatory proteins in biopsies from patients with IBD. Interestingly, this study also showed that CD55 was mainly expressed on the apical membrane of the epithelium, while CD46 was mainly found in the basolateral membrane (32). These findings contrast with those from studies showing that C3 and TCC were expressed on the apical membrane of IECs in patients with IBD and that CD59 was specifically reduced on IECs but not other cell types in patients with UC and CD, potentially causing complement-mediated destruction of the intestinal epithelium (33–35). These discrepancies may be explained by differences in antibody specificity, methods for tissue preparation, imaging techniques, and patients’ pathology. Regarding the latter explanation, Halstensen et al. showed that patients with CD exhibited significant deposition of C3b in the mucus layers, while patients with UC expressed IgG1 and C4c in addition to C3b, leading to the hypothesis that the classical pathway of complement activation may be more relevant in UC than CD (36). In agreement with these findings, Sünderhauf and collaborators found that C3 expression at the mRNA level was upregulated exclusively in colonic biopsies of patients with CD who were in remission and had no signs of inflammation compared with colon biopsies from healthy individuals and patients with UC (26). Interestingly, in colons from patients with CD with active inflammation, levels of C3 were comparable to those of their noninflamed counterparts but significantly higher than those of patients with UC and patients acting as controls, and there was a significant upregulation of CfB (Figure 3B). Notably, C5 levels were undetectable in the colonic biopsies, and although this study did not specifically assess protein expression level, this result confirms previous findings that argued against terminal activation of the complement pathway in IBD (32). Additional studies examining the potential differences in complement activation between patients with UC and CD found that patients with CD exhibited enhanced levels of C1q and C3 at the mRNA and protein levels and an increased number of IgM+ B cells compared with patients with UC irrespective of the inflammatory status (37).
Interestingly, Laufer and collaborators showed that C3 was limited to inflamed tissues, where its expression was observed in IECs and in a population of CD68+ macrophages that formed an inflammatory infiltrate in the crypts (38). In contrast, C4 mRNA was expressed diffusely by enterocytes both in patients with CD and in healthy individuals acting as controls and was also found in the mast cells in the submucosa of patients with CD, providing further evidence for the role of these cells as an additional source of complement in the intestine that may be relevant in health and disease (39). Higher C4 levels were also described by others in the jejunal fluids of patients with CD, where activated intestinal monocytes and macrophages were hypothesized to be the most likely source (40). Intriguingly, upregulation of the complement regulator CD55 was observed in patients with IBD, which may indicate that some components generated during the complement activation cascade may exert protective functions in intestinal inflammation (Figure 3B). However, based on the observation that CD55–/– mice were more susceptible to chemically induced colitis, the authors concluded that upregulation of CD55 may represent an attempt to block excessive complement activation (41).
There is also evidence for the involvement of the complement system in the regulation of the microbiome, which is highly relevant to IBD etiology. Specifically, Nissila et al. found that in pediatric patients with IBD the levels of C4B positively correlated with inflammation, and patients lacking C4B had enhanced microbiota diversity compared with patients with two copies of C4B. These findings point toward a potential role of C4B in exacerbating IBD-related dysbiosis via promoting excessive complement reactivity toward the intestinal flora (42).
Complement system in experimental models of intestinal inflammation. The evidence of complement involvement in IBD pathology in patients fueled multiple studies in animal models with the aim of dissecting the underlying mechanisms and identifying potential therapeutic avenues.
Sünderhauf et al. found that mice with active dextran sulphate sodium–induced (DSS-induced) colitis underwent significant transcriptional upregulation of C3, but not other complement components such as C2, Cfb, C5ar1, C5ar2, and C3ar, in the colon and in primary IECs. Interestingly, this study also reported that C3b fragments adhered to mucosa-associated bacteria, potentially favoring their phagocytosis by myeloid cells (26). These findings further suggest that complement activation may be secondary to disruption of the mucus layer that brings bacteria and epithelial cells into closer proximity, ultimately causing an inflammatory immune response.
Other studies found C3aR and C5aR upregulation in the inflamed tissues of DSS-treated mice, which led to more thorough investigations of their role in acute and chronic intestinal inflammation. Using full-body knockout mice, Wende and colleagues showed that C3aR was mildly detrimental during acute DSS-induced colitis in Th2-biased BALB/c but not in Th1-biased B6 strains of mice (43). In the absence of C3aR, BALB/c and B6 mice developed differing cytokine profiles during DSS-induced colitis, consistent with the described differences in T cell polarization between these two strains (43–45). These strain-specific findings are complicated to translate to human IBD. The same group also studied the effect of C5aR on intestinal inflammation, demonstrating that global lack of C5aR was protective against acute DSS-induced colitis (46). These findings are in agreement with reports showing that the use of C5aR antagonists is protective in acute models of colitis induced by DSS or by 2,4,6-trinitrobenzene sulfonic acid administration, which mimics the pathology of CD (47–49). Interestingly, in a model of chronic colitis, whereby multiple cycles of DSS administration are followed by recovery to more closely mirror the relapse/remission episodes observed in patients, Johswich and collaborators found that C5aR deficiency was detrimental and resulted in greater weight loss and intestinal pathology. During chronic inflammation, C5aR–/– mice exhibited increased IL-5 and reduced IL-4 and IFN-γ transcripts compared with WT mice (46). These differences may be at least in part due to the microbiome, which was not specifically examined in this context, or by the different roles that C5aR plays in multiple cell types depending on its subcellular locations (50–52). Furthermore, the study by Johswich also found that during chronic inflammation, C5aR–/– mice underwent a significant reduction in the levels of C3aR, despite these mice having an even higher number of myeloperoxidase-expressing neutrophils than in WT animals. This observation is particularly intriguing, as it suggests a potential direct or indirect role of C5aR on the expression of C3aR, which could exert a protective effect during chronic intestinal inflammation (46). Additionally, it is possible that, while C5aR deficiency protects from tissue damage during active disease, it is detrimental during the remission phase owing to its role in repair/regeneration. Accordingly, another study showed that in chronic colitis levels of C5aR were reduced in the colon during active inflammation but increased in the remission phase (53).
In line with the hypothesis that certain complement proteins may be protective against intestinal inflammation, C3 deficiency in mice was shown to promote the loss of E-cadherin, tight junctions, and ion channels and to increase inflammatory responses via the inducible nitric oxide synthase–mediated cyclooxygenase-2 pathway, with upregulation of TNF-α, IL-1 and IL-6 (54). Furthermore, C5–/–, C1q–/–, MBL–/–, C3–/–, fB–/–, and C6–/– mice all show increased susceptibility to acute and chronic DSS-induced colitis, further highlighting the important role of the complement system in the regulation of intestinal immune responses (53, 55–57). In the same studies, treatment with complement inhibitors (CR2-fH) targeted to sites of complement activation or inhibiting the alternative pathway effectively prevented excessive intestinal inflammation, reduced fibrosis, and lowered the number of infiltrating macrophages and B cells (53).
Overall, it remains unclear whether the complement system is a passenger or a driver in IBD. In a study aimed to characterize the contribution of rare genetic variants to IBD development, Shaw and collaborators found that mutations in complement C2, C3, and CFB were among the top 200 most significant common variants associated with disease in a cohort of 628 pediatric patients (58). Multiple mouse models lacking specific complement factors do not spontaneously develop intestinal inflammation, suggesting that mutations in complement genes may not be sufficient to drive IBD and that the condition requires a “second hit.” This is particularly intriguing considering that similar findings have been shown for mutations in other genes implicated in innate immunity and host microbiome interaction that are strongly associated with IBD. For instance, the great majority of individuals homozygous or heterozygous for NOD2 variants as well as Nod2-deficient mice housed under specific pathogen–free conditions do not develop CD without the presence of additional genetic hits or specific pathobionts (59–61).
-
Complement in colon cancer development, progression, and treatment
Activation of the complement system and its ability to tag, clear, lyse, and stimulate antibody-mediated cytotoxicity against foreign or altered cells has traditionally been seen an important aspect of immunosurveillance (62). As such, activation of the complement system has been described in multiple cancer types in human and preclinical models (63–65) (Table 2). However, owing to its ability to promote inflammation, complement can also play an active role in the development and progression of multiple cancers (66). Cancer cells not only have the ability to express higher levels of complement regulatory factors to evade complement-mediated killing, but in many instances they can produce and activate complement themselves, which suggests that complement is a double-edged sword in cancer and its roles go well beyond traditional immunosurveillance (67–70).
Complement system functions in colon cancer cells. In the context of colon cancer, there have been important insights from studies investigating the role of the complement system in the intestine using various colon carcinoma cell lines as surrogate of IECs. Andoh and collaborators reported that Caco-2 cells derived from human colon adenocarcinoma were able to generate functional C3, C4, and CfB and that the production of these complement factors could be further enhanced by stimulation with cytokines such as IL-1, TNF-α, IL-6, and IFN-γ (71). However, the functional role of complement production in cancer cells was not investigated. Other studies have shown that Caco-2, T84, and HT29 colon cancer cell lines can also respond to the complement split products C3a and C5a via expression of complement anaphylatoxin receptors on their apical membrane. Stimulation of these cell lines with C3a or C5a led to increased mRNA levels of CXCL2, CXCL8, CXCL11, CXCL10, and IL-8 following activation of the ERK pathway, which resulted in increased monolayer permeability (72, 73). However, the functional relevance of increased intestinal permeability is questionable in the context of colon cancer, where disruption of the epithelial barrier has already occurred. Moreover, other studies have shown that ERK activation is essential for the normal expression of key tight junction proteins and regulation of epithelial integrity (74, 75). Perhaps more functionally relevant in the context of cancer is the finding that C5a-induced IL-8 production enhanced the proliferation of colon cancer cell lines (73).
Additional findings support the role of complement in regulating cancer cell metabolism. C1Q binding protein (C1QBP) has been consistently found to be upregulated in multiple cancers (76–78). In patients with colon cancer, high C1QBP expression correlated with worse overall survival (OS) compared with patients with low C1QBP, and it was shown to interact with apolipoprotein A-I, potentially explaining the well-established antitumor effects of this high-density lipoprotein in human and preclinical models (79–81). Interestingly, Sünderhauf and collaborators found that C1QBP cleavage by caspase-1 prevented its import to the mitochondria, which resulted in reduced oxidative phosphorylation activity and enhanced glycolysis in HT29 colon cancer cell lines, ultimately increasing proliferation (Figure 3C). Although caspase-1 and C1QBP levels did not correlate with tumor stage, they were increased in human colon cancer tissues compared with the paired normal mucosa, demonstrating the potential relevance of inflammasome and complement interaction in patients with colon cancer (82).
Investigation of complement in human colon cancer. An analysis of The Cancer Genome Atlas datasets revealed that complement mutations occurred at high frequencies in multiple cancers and were associated with a robust reduction of OS in patients with colon cancer. Furthermore, the authors demonstrated that hypoxia supports the upregulation of CD55 on colon cancer cell lines, which results in protection from complement-mediated cytotoxicity, thus unraveling a novel crosstalk between complement and hypoxia. Interestingly, cancer-associated mutations resulting in higher CD55 expression correlated with worse disease-free survival (83). Prostaglandin E2 (PGE2), which is well known to promote colon cancer, was also shown to induce CD55 expression in cancer cells and in spontaneous models of intestinal tumorigenesis. Inhibition of CD55 was effective at restraining colon cancer development and metastases, further supporting the tumor-promoting role of this complement regulator (84–86) (Figure 3C).
Multiple studies found that certain complement mutations are more prevalent in specific ethnicities. For instance, loss-of-function mutations in complement C2 have been described to be more prevalent in populations with European ancestry, while loss-of-function mutations in complement C9 are more prevalent among those with Japanese ancestry (87, 88). Mutations in complement C5 and C6 appear to be highly prevalent in populations with African ethnicity in sub-Saharan Africa and among African American populations of the Southeastern region of the US, respectively (89, 90). In the case of C5, the single nucleotide polymorphism c.754G>A:p.A252T, which is common in the Western Cape population, is found in 7% of the African population with meningococcal disease. Despite affecting a small part of the full-length C5, the hypothesis is that this mutation may lead to longer folding times, ultimately affecting the C5 intracellular stability or secretion (89, 91). While it is well known that C5 and C6 mutations predispose to 10,000-fold higher frequencies of Neisseria meningococcal infections in these populations, their effect on colon cancer development, progression, and response to treatment remains largely understudied (92–94). This is a potentially interesting area of investigation, especially considering that populations of African ancestry are often diagnosed with more aggressive forms of colon cancer, which overall result in worse outcomes compared with populations of European ancestry (95, 96).
Mutations causing deficiencies in the lectin pathway of complement activation were initially hypothesized to predispose to increased risk of developing postoperative infections in patients with colon cancer, ultimately resulting in higher recurrence rates and mortality. However, studies by Ytting and collaborators showed that while the frequency of mannose binding lectin (MBL) mutations between healthy individuals and patients with colon cancer did not differ, the serum levels of MBL, MASP2 and the activity of MBL/MASP were significantly increased in the blood of patients with colon cancer, and high MASP-2 levels in serum proved to be an independent prognostic marker to predict recurrence and poor survival (97, 98).
Recognition of alteration of the complement system in human colon cancer also emerged from the first molecular characterization of colon cancer subtypes (99). That study identified four main colon cancer molecular subtypes (CMS1–4), with CMS1 cancers characterized by a distinct immune signature typical of microsatellite instability high (MSI-H) cancers, CMS2 cancers with MYC and WNT activation, CMS3 cancers with metabolic deregulation, and CMS4 tumors with a mesenchymal phenotype characterized by upregulation of TGF-β, angiogenic factors, and stromal infiltration. Interestingly, CMS4 cancers showed a distinct upregulation of the complement pathway. Although the complement genes responsible for driving the upregulation of this pathway in the CMS4 cancers were not specifically investigated in this study, the findings that patients with these cancers had worse OS and relapse-free survival compared with patients with other molecular subtypes suggest a detrimental role of the complement system in colon cancer prognosis (99). On the other hand, there is evidence that C3 is significantly downregulated in paired cancer and healthy colon tissue from patients with cancer compared with colon tissue from patients without cancer (100). Although this latter study did not assess the correlation between C3 expression and survival, the results suggest that the loss of C3 may favor tumor development. An analysis of publicly available datasets indicated that C3 expression was higher at the gene and protein level in patients with colon versus rectal cancer. Intriguingly, C3 expression in patients with colon cancer was strongly associated with antigen processing and presentation, neutrophil effector functions, B cell maturation, and humoral immune response pathways (101). Furthermore, C3 expression was associated with increased immunoscore, which is a positive prognostic factor in colon cancer and confers susceptibility to immune checkpoint blockade immunotherapy (ICI) (102–104). While the relationship between C3 expression and response was not investigated, in the above-mentioned study, C3 expression correlated with worse OS. These finding may appear contradictory. However, they are reminiscent of a trend previously described in the MSI-H colon cancers, in which the immune infiltrate is a prognostic factor associated with significantly better survival than microsatellite stable (MSS) colon cancers in the early stages but with worse survival in the metastatic setting (103, 105).
Complement anaphylatoxins in preclinical models of colon cancer. Complement anaphylatoxins have been extensively investigated in preclinical models of colorectal cancer because of their known role in promoting inflammation and recruitment of immunosuppressive cells of myeloid origin in the context of other malignancies. In a model of inflammation-induced colon cancer, Ning and collaborators observed reduced tumor development in the colons of C3–/– mice, suggesting a protumorigenic role of the complement system. Mechanistically, they showed that C5a release during tumor development stimulated the production of IL-1 by neutrophils, which acted in an autocrine and paracrine manner on mucosal myeloid cells and induced production of IL-17A, ultimately activating the STAT3/NF-κB–dependent pathway responsible for the hyperproliferation of the cancer cells (106) (Figure 3C). Piao et al. further showed that the C5a/C5aR axis promotes metastases formation in an experimental model of colorectal liver metastases by stimulating in the macrophages the production of monocyte chemoattractant protein (MCP-1) via the AKT signaling pathway. This resulted in increased recruitment of macrophages, neutrophils, and dendritic cells to the metastatic niche and promoted production of arginase, IL-10 and TGF-β, contributing to the development of a protumorigenic microenvironment and supporting the growth of metastases. In line with the findings by Ning et al., Piao et al. showed that C5aR expressed by the immune cells was mainly responsible for the observed effects. Additionally, while C5aR ablation significantly reduced the metastatic burden, it did not completely abrogate metastases, indicating that other mechanisms in addition to the C5a/C5aR axis are required for the establishment of colorectal liver metastases (107). Interestingly, in both studies, bone marrow chimera experiments demonstrated that only the complement expression in immune cells conferred a protumorigenic phenotype and promoted the formation of metastases. However, other studies have shown that cancer cell–intrinsic C5a/C5aR signaling may also play a role in intestinal tumorigenesis. Specifically, intracellular C5a stimulation of C5aR1 causes β-catenin stabilization and promotion of colon cancer (108). Along the same line, a study by Olcina and collaborators demonstrated that the C5a/C5aR axis in colon cancer cells plays a key role in their response to treatment. Specifically, by transplanting tumoroids that closely resembled the patient tumor microenvironment (TME), the authors found that C5aR1 expression was induced in the cancer cell as part of the stress response to radiotherapy. Inhibition of C5aR1 resulted in increased NF-κB–dependent apoptosis specifically in tumors, but not in normal tissues, and significantly improved the response to radiotherapy even in tumors with immunosuppressive features (Figure 3C). Notably, these effects were likely independent of T cells, as the reduced tumor growth was observed also in athymic mice, suggesting that C5aR1 inhibition plus radiotherapy may be an efficient combination treatment, even in tumors with immunosuppressive features and low T cell infiltration, which constitute most colon cancer cases (109).
Another study identified the crosstalk between dietary fats and the complement system as an important promoter of intestinal neoplasia. In this study, the authors took advantage of the well-established model APCMin/+ mice, which spontaneously develop polyps along in the intestine, as well as multiple mouse models with enhanced susceptibility to high-fat diet (HFD) treatment. Mechanistically, they showed that specific dietary fats such as hydrogenated coconut and corn oil but not olive oil activated the complement system, leading to the generation of C5a and upregulation of the proinflammatory cytokines MCP-1, IL-1β, IL-6, IL-23, TNF-α, and VEGF in adipose tissue, intestine, and blood, which correlated with increased tumorigenesis. Importantly, lean mice fed HFD presented increased inflammation and tumorigenesis, indicating that obesity and metabolic status were not the drivers of C5a-induced tumorigenesis. Pharmacologic and genetic targeting of C5aR prevented diet-induced local and systemic inflammation and significantly reduced polyp burden (110). Since the specific source of complement was not investigated in this study, it is not clear whether immune or nonimmune cells contributed to its production in response to HFD.
Using APCMin/+ mice, our group found that during spontaneous intestinal tumorigenesis there was an increase in protumorigenic low-density neutrophils with the ability to spontaneously release neutrophil extracellular traps (NETs). We showed that LPS, which was increased in the circulation of tumor-bearing mice as result of intestinal barrier dysfunction, induced activation of the complement cascade via the alternative pathway. These findings are in line with those of a previous study showing that the alternative pathway of complement activation was increased in patients with cancer, and this increase remained unaltered after surgery (111). Interestingly, LPS also induced upregulation of C3aR on neutrophils, which led to NETosis-dependent hypercoagulation and N2-phenotype (or tumor-promoting neutrophil) polarization, ultimately fueling tumorigenesis (Figure 3C). Ablation of C3aR in APCMin/+ mice (APCMin/+C3aR–/–) normalized the neutrophil counts, resolved the hypercoagulation, and significantly reduced tumor burden and NETs, demonstrating the relevance of this novel crosstalk between complement, neutrophils, and coagulation in intestinal tumorigenesis (112). Despite undergoing a significant reduction in small intestinal tumors, APCMin/+C3aR–/– mice showed a striking increase in number of colon tumors, which, although surprising, may be explained by the known differences between large and small intestine and by the potentially different functions of the C3a/C3aR axis in these specific compartments. These findings were not limited to the genetic model but could be recapitulated in an inflammation-driven model of colorectal cancer. Furthermore, we described increased permeability of the gut vascular barrier in APCMin/+C3aR–/– mice, which facilitated the translocation of bacteria to the liver to promote a premetastatic niche and favored the hematogenous dissemination of colon cancer cells with consequent development of metastases (113). By performing fecal microbiota transplantation, we proved that C3aR deficiency results in the development of a protumorigenic microbiota characterized by an increase in Bacteroidota (previously Bacteroidetes) and Gammaproteobacteria phyla. Furthermore, we showed high degree of immune infiltration in the colon tumors from C3aR–/– mice dominated by Th1, Th17, and cytotoxic T cell signatures. This immune signature conferred susceptibility to ICI and effectively reduced tumor growth in otherwise ICI-unresponsive APCMin/+ tumors.
By mining publicly available datasets, we found that C3aR downregulation occurs in subsets of human colon and rectal cancers independently of their MSI-H or MSS status and is driven by C3AR1 gene methylation. In line with our findings in the mouse models, in patients, reduced levels of C3aR expression correlated with increased accumulation of innate and adaptive immune responses in the TME, which supports antitumor immunity (114) (Figure 3C). It is interesting to note that, while during tumor development C3aR has a protective effect, in therapeutic settings the ablation of C3aR achieved comparable results to ablation of C5aR described in the context of ICI treatment in other cancers (115, 116).
Based on these findings, it is tempting to speculate that the C3a/C3aR axis regulates key physiologic functions of the large intestinal epithelium and, as such, loss or downregulation of C3aR could represent the Achilles’ heel of colon cancer. The complement system at mucosal surfaces such as the GI tract is essential to avoid overt inflammation by keeping immune responses to microbial flora in check. However, during tumor development and therapy, this regulatory mechanism may restrain the activation of immune responses and the C3a/C3aR axis can act as an immune checkpoint that prevents antitumor immunity in colon cancer.
-
Summary and future directions
A growing number of studies are contributing to deciphering the canonical and noncanonical functions of the complement system in the GI tract in homeostatic conditions, during inflammation, and in tumor development, progression, and treatment. The normal intestinal epithelium can produce its own complement factors, which can act in an autocrine or paracrine manner. Similarly, cancer cells can also produce and respond to complement factors and express complement inhibitory molecules. Overall, during intestinal inflammation and tumorigenesis the complement system can exert both protective and detrimental roles. The apparent contradictory results emerging from both human and preclinical studies may be explained by the distinct roles that the complement system plays not only in different cancers but also in different phases of the same cancer. While complement may participate in tumor destruction, it can also be exploited by cancer and immune cells to ultimately support tumor growth via different mechanisms. These paradoxical functions position the complement system within the framework of the 3C and 3E theory of immunoediting in cancer (117–119).
The discovery of the complosome, the identification of novel noncanonical functions of the complement proteins, and the availability of novel genetic models to dissect the function of specific complement factors in a cell-specific manner now make it possible to further explore this hypothesis and may lead to exciting developments in our understanding of role of the complement system in the GI tract.
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2025, Krieg et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2025;135(19):e188348. https://doi.org/10.1172/JCI188348.
-
References
- Horowitz A, et al. Paracellular permeability and tight junction regulation in gut health and disease. Nat Rev Gastroenterol Hepatol. 2023;20(7):417–432.
- Turpin W, et al. Increased intestinal permeability is associated with later development of Crohn’s disease. Gastroenterology. 2020;159(6):2092–2100.
- Torres J, et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology. 2020;159(1):96–104.
- Zeissig S, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56(1):61–72.
- Cerenius L, et al. Proteolytic cascades and their involvement in invertebrate immunity. Trends Biochem Sci. 2010;35(10):575–583.
- West EE, Kemper C. Complosome - the intracellular complement system. Nat Rev Nephrol. 2023;19(7):426–439.
- Hajishengallis G, et al. Novel mechanisms and functions of complement. Nat Immunol. 2017;18(12):1288–1298.
- Liszewski MK, et al. Complement’s hidden arsenal: New insights and novel functions inside the cell. Mol Immunol. 2017;84:2–9.
- Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171(3):715–727.
- Ricklin D, et al. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797.
- Dudkina NV, et al. Structure of the poly-C9 component of the complement membrane attack complex. Nat Commun. 2016;7:10588.
- Bharti R, et al. CD55 in cancer: Complementing functions in a non-canonical manner. Cancer Lett. 2022;551:215935.
- Saxena R, et al. Complement regulators as novel targets for anti-cancer therapy: A comprehensive review. Semin Immunol. 2025;77:101931.
- Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–740.
- Liszewski MK, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39(6):1143–1157.
- Schafer N, et al. Complement factor H-related 3 enhanced inflammation and complement activation in human RPE cells. Front Immunol. 2021;12:769242.
- Li Y, et al. Intracellular C3 prevents hepatic steatosis by promoting autophagy and very-low-density lipoprotein secretion. FASEB J. 2021;35(12):e22037.
- Friscic J, et al. The complement system drives local inflammatory tissue priming by metabolic reprogramming of synovial fibroblasts. Immunity. 2021;54(5):1002–1021.
- Kunz N, Kemper C. Complement has brains-do intracellular complement and immunometabolism cooperate in tissue homeostasis and behavior? Front Immunol. 2021;12:629986.
- King BC, et al. Complement component C3 is highly expressed in human pancreatic islets and prevents β cell death via ATG16L1 interaction and autophagy regulation. Cell Metab. 2019;29(1):202–210.
- Kulkarni HS, et al. Intracellular C3 protects human airway epithelial cells from stress-associated cell death. Am J Respir Cell Mol Biol. 2019;60(2):144–157.
- Reichhardt MP, Meri S. Intracellular complement activation-An alarm raising mechanism? Semin Immunol. 2018;38:54–62.
- Wu M, et al. Gut complement induced by the microbiota combats pathogens and spares commensals. Cell. 2024;187(4):897–913.
- Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31(4):154–163.
- Pope MR, et al. Complement regulates TLR4-mediated inflammatory responses during intestinal ischemia reperfusion. Mol Immunol. 2010;48(1-3):356–364.
- Sünderhauf A, et al. Regulation of epithelial cell expressed C3 in the intestine - Relevance for the pathophysiology of inflammatory bowel disease? Mol Immunol. 2017;90:227–238.
- Kopp ZA, et al. Do antimicrobial peptides and complement collaborate in the intestinal mucosa? Front Immunol. 2015;6:17.
- Ordas I, et al. Ulcerative colitis. Lancet. 2012;380(9853):1606–1619.
- Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365(18):1713–1725.
- Hodgson HJ, et al. C3 metabolism in ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1977;28(3):490–495.View this article via: PubMed Google Scholar
- Feinstein PA, et al. The alternate complement pathway in inflammatory bowel disease. Quantitation of the C3 proactivator (factor B) protein. Gastroenterology. 1976;70(2):181–185.
- Ueki T, et al. Distribution of activated complement, C3b, and its degraded fragments, iC3b/C3dg, in the colonic mucosa of ulcerative colitis (UC). Clin Exp Immunol. 1996;104(2):286–292.
- Scheinin T, et al. Decreased expression of protectin (CD59) in gut epithelium in ulcerative colitis and Crohn’s disease. Hum Pathol. 1999;30(12):1427–1430.
- Halstensen TS, et al. Epithelial deposits of immunoglobulin G1 and activated complement colocalise with the M(r) 40 kD putative autoantigen in ulcerative colitis. Gut. 1993;34(5):650–657.
- Halstensen TS, et al. Epithelial deposition of immunoglobulin G1 and activated complement (C3b and terminal complement complex) in ulcerative colitis. Gastroenterology. 1990;98(5 pt 1):1264–1271.
- Halstensen TS, et al. Surface epithelium related activation of complement differs in Crohn’s disease and ulcerative colitis. Gut. 1992;33(7):902–908.
- Preisker S, et al. Crohn’s disease patients in remission display an enhanced intestinal IgM+ B cell count in concert with a strong activation of the intestinal complement system. Cells. 2019;8(1):78.
- Laufer J, et al. Cellular localization of complement C3 and C4 transcripts in intestinal specimens from patients with Crohn’s disease. Clin Exp Immunol. 2000;120(1):30–37.
- Dvorak AM, et al. Crohn’s disease: transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations of mast cells, basophils, eosinophils, and the microvasculature. Hum Pathol. 1980;11(6):606–619.
- Ahrenstedt O, et al. Enhanced local production of complement components in the small intestines of patients with Crohn’s disease. N Engl J Med. 1990;322(19):1345–1349.
- Lin F, et al. Decay-accelerating factor deficiency increases susceptibility to dextran sulfate sodium-induced colitis: role for complement in inflammatory bowel disease. J Immunol. 2004;172(6):3836–3841.
- Nissila E, et al. C4B gene influences intestinal microbiota through complement activation in patients with paediatric-onset inflammatory bowel disease. Clin Exp Immunol. 2017;190(3):394–405.
- Wende E, et al. The complement anaphylatoxin C3a receptor (C3aR) contributes to the inflammatory response in dextran sulfate sodium (DSS)-induced colitis in mice. PLoS One. 2013;8(4):e62257.
- Alex P, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15(3):341–352.
- Egger B, et al. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62(4):240–248.
- Johswich K, et al. Role of the C5a receptor (C5aR) in acute and chronic dextran sulfate-induced models of inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(12):1812–1823.
- Cui CS, et al. Colon-targeted complement C5a1 receptor inhibition using pH-sensitive nanoparticles ameliorates experimental colitis. Br J Pharmacol. 2025;182(16):3852–3869.
- Woodruff TM, et al. Increased potency of a novel complement factor 5a receptor antagonist in a rat model of inflammatory bowel disease. J Pharmacol Exp Ther. 2005;314(2):811–817.
- Woodruff TM, et al. A potent human C5a receptor antagonist protects against disease pathology in a rat model of inflammatory bowel disease. J Immunol. 2003;171(10):5514–5520.
- Arbore G, et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4+ T cells. Science. 2016;352(6292):aad1210.
- Niyonzima N, et al. Mitochondrial C5aR1 activity in macrophages controls IL-1β production underlying sterile inflammation. Sci Immunol. 2021;6(66):eabf2489.
- O’Brien RM, et al. Thinking inside the box: intracellular roles for complement system proteins come into focus. Br J Cancer. 2023;128(2):165–167.
- Elvington M, et al. Regulation of the alternative pathway of complement modulates injury and immunity in a chronic model of dextran sulphate sodium-induced colitis. Clin Exp Immunol. 2015;179(3):500–508.
- Choi YJ, et al. Promotion of the inflammatory response in mid colon of complement component 3 knockout mice. Sci Rep. 2022;12(1):1700.
- Ding P, et al. Complement component 6 deficiency increases susceptibility to dextran sulfate sodium-induced murine colitis. Immunobiology. 2016;221(11):1293–1303.
- Schepp-Berglind J, et al. Complement-dependent injury and protection in a murine model of acute dextran sulfate sodium-induced colitis. J Immunol. 2012;188(12):6309–6318.
- Deguchi Y, et al. Development of dextran sulfate sodium-induced colitis is aggravated in mice genetically deficient for complement C5. Int J Mol Med. 2005;16(4):605–608.View this article via: PubMed Google Scholar
- Shaw KA, et al. Genetic variants and pathways implicated in a pediatric inflammatory bowel disease cohort. Genes Immun. 2019;20(2):131–142.
- Brant SR, et al. A population-based case-control study of CARD15 and other risk factors in Crohn’s disease and ulcerative colitis. Am J Gastroenterol. 2007;102(2):313–323.
- Kim YG, et al. Cutting edge: Crohn’s disease-associated Nod2 mutation limits production of proinflammatory cytokines to protect the host from Enterococcus faecalis-induced lethality. J Immunol. 2011;187(6):2849–2852.
- Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307(5710):731–734.
- Dechant M, et al. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res. 2008;68(13):4998–5003.
- Rutkowski MJ, et al. Cancer and the complement cascade. Mol Cancer Res. 2010;8(11):1453–1465.
- Roumenina LT, et al. Context-dependent roles of complement in cancer. Nat Rev Cancer. 2019;19(12):698–715.
- Reis ES, et al. Complement in cancer: untangling an intricate relationship. Nat Rev Immunol. 2018;18(1):5–18.
- Markiewski MM, Lambris JD. Is complement good or bad for cancer patients? A new perspective on an old dilemma. Trends Immunol. 2009;30(6):286–292.
- Lu Y, Hu XB. C5a stimulates the proliferation of breast cancer cells via Akt-dependent RGC-32 gene activation. Oncol Rep. 2014;32(6):2817–2823.
- Markiewski MM, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9(11):1225–1235.
- Fishelson Z, et al. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40(2-4):109–123.
- Yu J, et al. Protection of human breast cancer cells from complement-mediated lysis by expression of heterologous CD59. Clin Exp Immunol. 1999;115(1):13–18.
- Andoh A, et al. Differential cytokine regulation of complement C3, C4, and factor B synthesis in human intestinal epithelial cell line, Caco-2. J Immunol. 1993;151(8):4239–4247.
- McCarthy JD, et al. The anaphylatoxin C3a primes model colonic epithelial cells for expression of inflammatory mediators through Gαi. Mol Immunol. 2018;103:125–132.
- Cao Q, et al. Human colonic epithelial cells detect and respond to C5a via apically expressed C5aR through the ERK pathway. Am J Physiol Cell Physiol. 2012;302(12):C1731–C1740.
- Lipschutz JH, et al. Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells. J Biol Chem. 2005;280(5):3780–3788.
- Wang Y, et al. Activation of ERK1/2 MAP kinase pathway induces tight junction disruption in human corneal epithelial cells. Exp Eye Res. 2004;78(1):125–136.
- Saha SK, et al. Systematic multiomics analysis of alterations in C1QBP mRNA expression and relevance for clinical outcomes in cancers. J Clin Med. 2019;8(4):513.
- Dembitzer FR, et al. gC1qR expression in normal and pathologic human tissues: differential expression in tissues of epithelial and mesenchymal origin. J Histochem Cytochem. 2012;60(6):467–474.
- Chen YB, et al. Increased expression of hyaluronic acid binding protein 1 is correlated with poor prognosis in patients with breast cancer. J Surg Oncol. 2009;100(5):382–386.
- Kim K, et al. C1QBP is upregulated in colon cancer and binds to apolipoprotein A-I. Exp Ther Med. 2017;13(5):2493–2500.
- Su F, et al. HDL mimetics inhibit tumor development in both induced and spontaneous mouse models of colon cancer. Mol Cancer Ther. 2012;11(6):1311–1319.
- van Duijnhoven FJ, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut. 2011;60(8):1094–1102.
- Sünderhauf A, et al. GC1qR cleavage by caspase-1 drives aerobic glycolysis in tumor cells. Front Oncol. 2020;10:575854.
- Olcina MM, et al. Mutations in an innate immunity pathway are associated with poor overall survival outcomes and hypoxic signaling in cancer. Cell Rep. 2018;25(13):3721–3732.
- Tang G, et al. Knockdown of membrane-bound complement regulatory proteins suppresses colon cancer growth in mice through inducing tumor cell apoptosis. Int Immunopharmacol. 2023;114:109450.
- Holla VR, et al. Prostaglandin E2 regulates the complement inhibitor CD55/decay-accelerating factor in colorectal cancer. J Biol Chem. 2005;280(1):476–483.
- Bernet-Camard MF, et al. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut. 1996;38(2):248–253.
- Fukumori Y, et al. A high incidence of C9 deficiency among healthy blood donors in Osaka, Japan. Int Immunol. 1989;1(1):85–89.
- Rynes RI, et al. Deficiency of the second complement component association with the HLA haplotype A10, B18 in a normal population. Ann Rheum Dis. 1982;41(1):93–96.
- Franco-Jarava C, et al. Complement factor 5 (C5) p.A252T mutation is prevalent in, but not restricted to, sub-Saharan Africa: implications for the susceptibility to meningococcal disease. Clin Exp Immunol. 2017;189(2):226–231.
- Zhu Z, et al. High prevalence of complement component C6 deficiency among African-Americans in the south-eastern USA. Clin Exp Immunol. 2000;119(2):305–310.
- Owen EP, et al. A complement C5 gene mutation, c.754G>A:p.A252T, is common in the Western Cape, South Africa and found to be homozygous in seven percent of Black African meningococcal disease cases. Mol Immunol. 2015;64(1):170–176.
- Lehner PJ, et al. Meningococcal septicaemia in a C6-deficient patient and effects of plasma transfusion on lipopolysaccharide release. Lancet. 1992;340(8832):1379–1381.
- Orren A, et al. Deficiency of the sixth component of complement and susceptibility to Neisseria meningitidis infections: studies in 10 families and five isolated cases. Immunology. 1987;62(2):249–253.View this article via: PubMed Google Scholar
- Nicholson A, Lepow IH. Host defense against Neisseria meningitidis requires a complement-dependent bactericidal activity. Science. 1979;205(4403):298–299.
- Holowatyj AN, et al. Racial/ethnic disparities in survival among patients with young-onset colorectal cancer. J Clin Oncol. 2016;34(18):2148–2156.
- Wallace K, et al. Racial disparities in advanced-stage colorectal cancer survival. Cancer Causes Control. 2013;24(3):463–471.View this article via: PubMed Google Scholar
- Ytting H, et al. Serum mannan-binding lectin-associated serine protease 2 levels in colorectal cancer: relation to recurrence and mortality. Clin Cancer Res. 2005;11(4):1441–1446.
- Ytting H, et al. Increased activity of the mannan-binding lectin complement activation pathway in patients with colorectal cancer. Scand J Gastroenterol. 2004;39(7):674–679.
- Guinney J, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356.
- Lian J, et al. Aberrant gene expression profile of unaffected colon mucosa from patients with unifocal colon polyp. Med Sci Monit. 2015;21:3935–3940.
- Liang JZ, et al. Comparative proteome identifies complement component 3-mediated immune response as key difference of colon adenocarcinoma and rectal adenocarcinoma. Front Oncol. 2020;10:617890.
- Roth AD, et al. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst. 2012;104(21):1635–1646.
- Popat S, et al. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609–618.
- Le DT, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520.
- Tran B, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117(20):4623–4632.
- Ning C, et al. Complement activation promotes colitis-associated carcinogenesis through activating intestinal IL-1β/IL-17A axis. Mucosal Immunol. 2015;8(6):1275–1284.
- Piao C, et al. Complement 5a enhances hepatic metastases of colon cancer via monocyte chemoattractant protein-1-mediated inflammatory cell infiltration. J Biol Chem. 2015;290(17):10667–10676.
- Ding P, et al. Intracellular complement C5a/C5aR1 stabilizes β-catenin to promote colorectal tumorigenesis. Cell Rep. 2022;39(9):110851.
- Beach C, et al. Improving radiotherapy in immunosuppressive microenvironments by targeting complement receptor C5aR1. J Clin Invest. 2024;134(16):e185067.
- Doerner SK, et al. High-fat diet-induced complement activation mediates intestinal inflammation and neoplasia, independent of obesity. Mol Cancer Res. 2016;14(10):953–965.
- Baatrup G, et al. Perioperative functional activity of the alternative pathway of complement in patients with colonic cancer. Eur J Surg. 1999;165(10):962–965.
- Guglietta S, et al. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat Commun. 2016;7:11037.
- Bertocchi A, et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. 2021;39(5):708–724.
- Krieg C, et al. Complement downregulation promotes an inflammatory signature that renders colorectal cancer susceptible to immunotherapy. J Immunother Cancer. 2022;10(9):e004717.
- Ajona D, et al. A combined PD-1/C5a blockade synergistically protects against lung cancer growth and metastasis. Cancer Discov. 2017;7(7):694–703.
- Wang Y, et al. Autocrine complement inhibits IL10-dependent T-cell-mediated antitumor immunity to promote tumor progression. Cancer Discov. 2016;6(9):1022–1035.
- Artero MR, et al. Complement and the hallmarks of cancer. Semin Immunol. 2025;78:101950.
- Dunn GP, et al. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148.
- Mittal D, et al. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25.
- [No authors listed]. Correction: Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2023;72(12):e7.
- Birchenough GM, et al. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015;8(4):712–719.
- Etienne-Mesmin L, et al. Experimental models to study intestinal microbes-mucus interactions in health and disease. FEMS Microbiol Rev. 2019;43(5):457–489.
- Johansson ME, et al. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A. 2011;108 Suppl 1(suppl 1):4659–4665.
- Allaire JM, et al. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39(9):677–696.
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10(2):131–144.
- Price AE, et al. A map of toll-like receptor expression in the intestinal epithelium reveals distinct spatial, cell type-specific, and temporal patterns. Immunity. 2018;49(3):560–575.
- Rakoff-Nahoum S, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241.
- Yu S, Gao N. Compartmentalizing intestinal epithelial cell toll-like receptors for immune surveillance. Cell Mol Life Sci. 2015;72(17):3343–3353.
- Law SK, Dodds AW. The internal thioester and the covalent binding properties of the complement proteins C3 and C4. Protein Sci. 1997;6(2):263–274.
- Cochrane CG, Muller-Eberhard HJ. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968;127(2):371–386.
- Strainic MG, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28(3):425–435.
- Seya T, et al. CD46 (membrane cofactor protein of complement, measles virus receptor): structural and functional divergence among species (review). Int J Mol Med. 1998;1(5):809–816.View this article via: PubMed Google Scholar
- Iida K, Nussenzweig V. Complement receptor is an inhibitor of the complement cascade. J Exp Med. 1981;153(5):1138–1150.
- Nordahl EA, et al. Activation of the complement system generates antibacterial peptides. Proc Natl Acad Sci U S A. 2004;101(48):16879–16884.
- Fayyazi A, et al. C5a receptor and interleukin-6 are expressed in tissue macrophages and stimulated keratinocytes but not in pulmonary and intestinal epithelial cells. Am J Pathol. 1999;154(2):495–501.
- Zwirner J, et al. Expression of the anaphylatoxin C5a receptor in non-myeloid cells. Mol Immunol. 1999;36(13-14):877–884.
- Matsumoto N, et al. C3a enhances the formation of intestinal organoids through C3aR1. Front Immunol. 2017;8:1046.
- Quell KM, et al. Monitoring C3aR expression using a floxed tdTomato-C3aR reporter knock-in mouse. J Immunol. 2017;199(2):688–706.
- Gunji N, et al. Upregulation of complement C1q reflects mucosal regeneration in a mouse model of colitis. Med Mol Morphol. 2021;54(2):87–94.
- Zhang X, et al. Inhibition of C3a/C3aR axis in diverse stages of ulcerative colitis affected the prognosis of UC by modulating the pyroptosis and expression of caspase-11. Inflammation. 2020;43(6):2128–2136.
- Jain U, et al. The C5a receptor antagonist PMX205 ameliorates experimentally induced colitis associated with increased IL-4 and IL-10. Br J Pharmacol. 2013;168(2):488–501.
- Chen G, et al. Blockade of complement activation product C5a activity using specific antibody attenuates intestinal damage in trinitrobenzene sulfonic acid induced model of colitis. Lab Invest. 2011;91(3):472–483.
- Downs-Canner S, et al. Complement inhibition: a novel form of immunotherapy for colon cancer. Ann Surg Oncol. 2016;23(2):655–662.
- Surace L, et al. Complement is a central mediator of radiotherapy-induced tumor-specific immunity and clinical response. Immunity. 2015;42(4):767–777.
- Beach C, et al. Improving radiotherapy in immunosuppressive microenvironments by targeting complement receptor C5aR1. J Clin Invest. 2023;133(23):e168277.
- Ding P, et al. C5aR1 is a master regulator in Colorectal Tumorigenesis via Immune modulation. Theranostics. 2020;10(19):8619–8632.
-
Version history
- Version 1 (October 1, 2025): Electronic publication
Article tools
-
View PDF

- Download citation information
- Send a comment
- Terms of use
- Standard abbreviations
- Need help? Email the journal
Review Series
Complement Biology and Therapeutics
-
Role of local complement activation in kidney fibrosis and repair
Didier Portilla et al. -
Emerging roles for complement in lung transplantation
Hrishikesh S. Kulkarni et al. -
Friend or foe: assessing the value of animal models for facilitating clinical breakthroughs in complement research
Felix Poppelaars et al. -
The complement system in intestinal inflammation and cancer
Carsten Krieg et al. -
Gut complement system: a new frontier in microbiota-host communication and intestinal homeostasis
Xianbin Tian et al. -
The secret life of complement: challenges and opportunities in exploring functions of the complosome in disease
Tilo Freiwald et al. -
The complement system and kidney cancer: pathogenesis to clinical applications
Ravikumar Aalinkeel et al. -
Complement’s involvement in allergic Th2 immunity: a cross-barrier perspective
Sarah A. Thomas et al. -
Chronic kidney disease enhances alternative pathway activity: a new paradigm
Diana I. Jalal et al. -
The multiverse of CD46 and oncologic interactions
M. Kathryn Liszewski et al.
Metrics



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)





