Advertisement
Clinical Research and Public Health Free access | 10.1172/JCI81229
Outcomes of acute leukemia patients transplanted with naive T cell–depleted stem cell grafts
Marie Bleakley,1,2 Shelly Heimfeld,1 Keith R. Loeb,1,3 Lori A. Jones,4 Colette Chaney,1 Stuart Seropian,5 Ted A. Gooley,1 Franziska Sommermeyer,1 Stanley R. Riddell,1,6,7 and Warren D. Shlomchik5,8
1Fred Hutchinson Cancer Research Center (FHCRC), Seattle, Washington, USA.
2Department of Pediatrics and
3Department of Pathology, University of Washington, Seattle, Washington, USA.
4Seattle Cancer Care Alliance, Seattle, Washington, USA.
5Yale University School of Medicine (YUSM), New Haven, Connecticut, USA.
6Department of Medicine, University of Washington, Seattle, Washington, USA.
7Institute for Advanced Study, Technical University of Munich, Munich, Germany.
8University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Address correspondence to: Marie Bleakley, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, Washington 98109, USA. Phone: 206.667.6572; E-mail: mbleakle@fredhutch.org.
Authorship note: Stanley R. Riddell and Warren D. Shlomchik are co–senior authors.
Find articles by Bleakley, M. in: PubMed | Google Scholar
1Fred Hutchinson Cancer Research Center (FHCRC), Seattle, Washington, USA.
2Department of Pediatrics and
3Department of Pathology, University of Washington, Seattle, Washington, USA.
4Seattle Cancer Care Alliance, Seattle, Washington, USA.
5Yale University School of Medicine (YUSM), New Haven, Connecticut, USA.
6Department of Medicine, University of Washington, Seattle, Washington, USA.
7Institute for Advanced Study, Technical University of Munich, Munich, Germany.
8University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Address correspondence to: Marie Bleakley, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, Washington 98109, USA. Phone: 206.667.6572; E-mail: mbleakle@fredhutch.org.
Authorship note: Stanley R. Riddell and Warren D. Shlomchik are co–senior authors.
Find articles by Heimfeld, S. in: PubMed | Google Scholar
1Fred Hutchinson Cancer Research Center (FHCRC), Seattle, Washington, USA.
2Department of Pediatrics and
3Department of Pathology, University of Washington, Seattle, Washington, USA.
4Seattle Cancer Care Alliance, Seattle, Washington, USA.
5Yale University School of Medicine (YUSM), New Haven, Connecticut, USA.
6Department of Medicine, University of Washington, Seattle, Washington, USA.
7Institute for Advanced Study, Technical University of Munich, Munich, Germany.
8University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Address correspondence to: Marie Bleakley, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, Washington 98109, USA. Phone: 206.667.6572; E-mail: mbleakle@fredhutch.org.
Authorship note: Stanley R. Riddell and Warren D. Shlomchik are co–senior authors.
Find articles by Loeb, K. in: PubMed | Google Scholar
1Fred Hutchinson Cancer Research Center (FHCRC), Seattle, Washington, USA.
2Department of Pediatrics and
3Department of Pathology, University of Washington, Seattle, Washington, USA.
4Seattle Cancer Care Alliance, Seattle, Washington, USA.
5Yale University School of Medicine (YUSM), New Haven, Connecticut, USA.
6Department of Medicine, University of Washington, Seattle, Washington, USA.
7Institute for Advanced Study, Technical University of Munich, Munich, Germany.
8University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Address correspondence to: Marie Bleakley, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, Washington 98109, USA. Phone: 206.667.6572; E-mail: mbleakle@fredhutch.org.
Authorship note: Stanley R. Riddell and Warren D. Shlomchik are co–senior authors.
Find articles by Jones, L. in: PubMed | Google Scholar
1Fred Hutchinson Cancer Research Center (FHCRC), Seattle, Washington, USA.
2Department of Pediatrics and
3Department of Pathology, University of Washington, Seattle, Washington, USA.
4Seattle Cancer Care Alliance, Seattle, Washington, USA.
5Yale University School of Medicine (YUSM), New Haven, Connecticut, USA.
6Department of Medicine, University of Washington, Seattle, Washington, USA.
7Institute for Advanced Study, Technical University of Munich, Munich, Germany.
8University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Address correspondence to: Marie Bleakley, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, Washington 98109, USA. Phone: 206.667.6572; E-mail: mbleakle@fredhutch.org.
Authorship note: Stanley R. Riddell and Warren D. Shlomchik are co–senior authors.
Find articles by Chaney, C. in: PubMed | Google Scholar
1Fred Hutchinson Cancer Research Center (FHCRC), Seattle, Washington, USA.
2Department of Pediatrics and
3Department of Pathology, University of Washington, Seattle, Washington, USA.
4Seattle Cancer Care Alliance, Seattle, Washington, USA.
5Yale University School of Medicine (YUSM), New Haven, Connecticut, USA.
6Department of Medicine, University of Washington, Seattle, Washington, USA.
7Institute for Advanced Study, Technical University of Munich, Munich, Germany.
8University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Address correspondence to: Marie Bleakley, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, Washington 98109, USA. Phone: 206.667.6572; E-mail: mbleakle@fredhutch.org.
Authorship note: Stanley R. Riddell and Warren D. Shlomchik are co–senior authors.
Find articles by Seropian, S. in: PubMed | Google Scholar
1Fred Hutchinson Cancer Research Center (FHCRC), Seattle, Washington, USA.
2Department of Pediatrics and
3Department of Pathology, University of Washington, Seattle, Washington, USA.
4Seattle Cancer Care Alliance, Seattle, Washington, USA.
5Yale University School of Medicine (YUSM), New Haven, Connecticut, USA.
6Department of Medicine, University of Washington, Seattle, Washington, USA.
7Institute for Advanced Study, Technical University of Munich, Munich, Germany.
8University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Address correspondence to: Marie Bleakley, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, Washington 98109, USA. Phone: 206.667.6572; E-mail: mbleakle@fredhutch.org.
Authorship note: Stanley R. Riddell and Warren D. Shlomchik are co–senior authors.
Find articles by Gooley, T. in: PubMed | Google Scholar
1Fred Hutchinson Cancer Research Center (FHCRC), Seattle, Washington, USA.
2Department of Pediatrics and
3Department of Pathology, University of Washington, Seattle, Washington, USA.
4Seattle Cancer Care Alliance, Seattle, Washington, USA.
5Yale University School of Medicine (YUSM), New Haven, Connecticut, USA.
6Department of Medicine, University of Washington, Seattle, Washington, USA.
7Institute for Advanced Study, Technical University of Munich, Munich, Germany.
8University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Address correspondence to: Marie Bleakley, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, Washington 98109, USA. Phone: 206.667.6572; E-mail: mbleakle@fredhutch.org.
Authorship note: Stanley R. Riddell and Warren D. Shlomchik are co–senior authors.
Find articles by Sommermeyer, F. in: PubMed | Google Scholar
1Fred Hutchinson Cancer Research Center (FHCRC), Seattle, Washington, USA.
2Department of Pediatrics and
3Department of Pathology, University of Washington, Seattle, Washington, USA.
4Seattle Cancer Care Alliance, Seattle, Washington, USA.
5Yale University School of Medicine (YUSM), New Haven, Connecticut, USA.
6Department of Medicine, University of Washington, Seattle, Washington, USA.
7Institute for Advanced Study, Technical University of Munich, Munich, Germany.
8University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Address correspondence to: Marie Bleakley, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, Washington 98109, USA. Phone: 206.667.6572; E-mail: mbleakle@fredhutch.org.
Authorship note: Stanley R. Riddell and Warren D. Shlomchik are co–senior authors.
Find articles by Riddell, S. in: PubMed | Google Scholar
1Fred Hutchinson Cancer Research Center (FHCRC), Seattle, Washington, USA.
2Department of Pediatrics and
3Department of Pathology, University of Washington, Seattle, Washington, USA.
4Seattle Cancer Care Alliance, Seattle, Washington, USA.
5Yale University School of Medicine (YUSM), New Haven, Connecticut, USA.
6Department of Medicine, University of Washington, Seattle, Washington, USA.
7Institute for Advanced Study, Technical University of Munich, Munich, Germany.
8University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Address correspondence to: Marie Bleakley, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, Washington 98109, USA. Phone: 206.667.6572; E-mail: mbleakle@fredhutch.org.
Authorship note: Stanley R. Riddell and Warren D. Shlomchik are co–senior authors.
Find articles by Shlomchik, W. in: PubMed | Google Scholar
Authorship note: Stanley R. Riddell and Warren D. Shlomchik are co–senior authors.
Published June 8, 2015 - More info
J Clin Invest. 2015;125(7):2677–2689. https://doi.org/10.1172/JCI81229.
Copyright © 2015, American Society for Clinical Investigation
Received: February 4, 2015; Accepted: April 30, 2015
-
Abstract
BACKGROUND. Graft-versus-host disease (GVHD) is a major cause of morbidity and mortality following allogeneic hematopoietic stem cell transplantation (HCT). In mice, naive T cells (TN) cause more severe GVHD than memory T cells (TM). We hypothesized that selective depletion of TN from human allogeneic peripheral blood stem cell (PBSC) grafts would reduce GVHD and provide sufficient numbers of hematopoietic stem cells and TM to permit hematopoietic engraftment and the transfer of pathogen-specific T cells from donor to recipient, respectively.
METHODS. In a single-arm clinical trial, we transplanted 35 patients with high-risk leukemia with TN-depleted PBSC grafts following conditioning with total body irradiation, thiotepa, and fludarabine. GVHD prophylactic management was with tacrolimus immunosuppression alone. Subjects received CD34-selected PBSCs and a defined dose of TM purged of CD45RA+ TN. Primary and secondary objectives included engraftment, acute and chronic GVHD, and immune reconstitution.
RESULTS. All recipients of TN-depleted PBSCs engrafted. The incidence of acute GVHD was not reduced; however, GVHD in these patients was universally corticosteroid responsive. Chronic GVHD was remarkably infrequent (9%; median follow-up 932 days) compared with historical rates of approximately 50% with T cell–replete grafts. TM in the graft resulted in rapid T cell recovery and transfer of protective virus-specific immunity. Excessive rates of infection or relapse did not occur and overall survival was 78% at 2 years.
CONCLUSION. Depletion of TN from stem cell allografts reduces the incidence of chronic GVHD, while preserving the transfer of functional T cell memory.
TRIAL REGISTRATION. ClinicalTrials.gov (NCT 00914940).
FUNDING. NIH, Burroughs Wellcome Fund, Leukemia and Lymphoma Society, Damon Runyon Cancer Research Foundation, and Richard Lumsden Foundation.
-
Introduction
Allogeneic hematopoietic stem cell transplantation (HCT) is often curative for patients with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and other hematologic malignancies (1, 2). Donor T cells in the transplanted graft contribute to successful HCT by promoting the establishment of donor hematopoiesis, transferring pathogen-specific immunity, and mediating a graft-versus-leukemia (GVL) effect. Unfortunately in HLA-matched HCT, donor T cells that recognize recipient minor histocompatibility (H) antigens are also central to the development of graft-versus-host disease (GVHD), which remains a major cause of morbidity and mortality after HCT (3, 4).
To prevent or diminish the severity of GVHD in patients receiving T cell–replete allografts, patients receive several months of pharmacologic immunosuppression with calcineurin inhibitor–based regimens. Nonetheless, 30% to 70% and 40% to 63% of patients who receive HLA-matched related donor (MRD) grafts develop acute GVHD (aGVHD) and chronic GVHD (cGVHD), respectively (5–7). GVHD can be substantially reduced by nonselective removal of T cells from the stem cell graft or by early in vivo administration of T cell–depleting antibodies (7–9). Unfortunately, pan–T cell depletion (TCD) is complicated by delayed immune reconstitution and an increased frequency of opportunistic infections (10–12).
αβ T cells exist in the blood, secondary lymphoid organs, and tissues as distinct naive (TN), effector (TE), and memory (TM) subsets that can be distinguished by alterations in cell surface phenotype that occur as a consequence of activation with cognate antigen (13). The CD45RA+CD62L+ TN subset is antigen inexperienced and has a more diverse T cell receptor (TCR) repertoire than TM (14, 15). After antigen-driven activation, TN are induced to clonally expand and differentiate into short-lived effector cells and subsets of long-lived TM that protect the host from reinfection and include CD45RO+CD62L+ central memory (TCM), CD45RO+CD62L– effector memory (TEM), and CD45RO+CD62L–CD69+ tissue-resident memory (TRM) cells. CD4+ FOXP3+ Tregs are a separate subset of T cells that is derived by both thymic and extrathymic pathways and suppresses autoimmunity (16). Based on knowledge of the phenotype, repertoire, and reactivity of T cell subsets, we predicted that a strategy for engineering allogeneic stem cell grafts might be designed to separate the beneficial functions of T cells from detrimental GVHD after HCT. In mouse allogeneic HCT performed without immunosuppression, we and others showed that TN caused severe GVHD, TCM induced milder GVHD, and TEM did not cause significant GVHD (17–23). Importantly, TM transferred antipathogen immunity and had GVL activity in these models (17, 22, 24). Mechanistic studies demonstrated that TCR repertoire–independent and –dependent differences between TN and TM subsets contributed to differences in GVHD induction (20, 25–27). Consistent with the results in mice, we found, using sensitive in vitro assays, that the frequency of human CD8+ T cells specific for minor H antigens was at least 5- to 20-fold higher in TN than TM (28).
To test the hypothesis that removing TN from human allogeneic HCT grafts would reduce serious GVHD and allow the transfer of functional pathogen-specific immunity, we developed what we believe to be a novel graft-engineering strategy in which TN were selectively depleted from granulocyte colony-stimulating factor–mobilized peripheral blood stem cells (PBSCs) using immunomagnetic selection with a clinical-grade iron-dextran bead conjugated to a monoclonal antibody targeting CD45RA, which is expressed on the cell surface of all TN but is absent on TCM and most TM (29). We then designed a single-arm phase II trial in which patients with high-risk acute leukemia or advanced myelodysplastic syndrome (MDS) received a TN-depleted stem cell graft from a HLA-MRD. The use of a MRD provided a margin of safety if unforeseen problems occurred during graft engineering by allowing immediate access to additional granulocyte colony-stimulating factor–mobilized donor PBSCs. We used myeloablative conditioning composed of fludarabine, thiotepa, and total body irradiation (TBI; 1,320 cGy) (30). This regimen was chosen because it results in acceptable engraftment rates in the context of complete TCD HCT and should also permit engraftment following TN-depleted HCT without the need for antibody therapy before HCT with antithymocyte globulin or anti–T cell mAb, which could lead to depletion of TM in TN-depleted grafts (30). Tacrolimus alone was used as GVHD prophylaxis and was tapered after 50 days in the absence of GVHD. Here, we report the clinical outcomes of 35 consecutive patients treated in this clinical trial.
-
Results
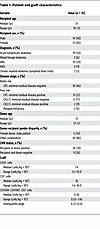
Patient characteristics and graft engineering. The patient characteristics are listed in Table 1. Of 108 patients aged 14 to 55 years with an HLA-MRD (>13 years old) who were evaluated for HCT for the treatment of acute leukemia or advanced MDS during the study period (December 2009 to May 2014), 35 were allocated to HCT in the TN depletion clinical trial and treated accordingly (Figure 1). The major reasons that other patients were not allocated to the trial included allocation to a non-TBI–containing myeloablative HCT at the discretion of the transplant attending physician (in most cases, patients with AML or MDS with better-risk disease and/or comorbidities); allocation to a nonmyeloablative regimen; or allocation to transplantation on a standard myeloablative treatment plan with cyclophosphamide and TBI conditioning. Patients allocated to cyclophosphamide and TBI included patients that lacked insurance coverage for a clinical trial, were unwilling to consent to the trial of TN depletion, or were precluded for participating for logistical reasons or because they did not meet eligibility criteria for the trial.
 Figure 1
Figure 1Consort diagram of phase II nonrandomized clinical trial. Number of patients assessed for eligibility and excluded or allocated to the trial, treated, followed, and analyzed. Patients with AML, ALL, or MDS could be allocated to nonmyeloablative HCT or to less intensive, non-TBI myeloablative HCT if the attending physician determined that their disease status required less intensive conditioning or if the patient had significant co-morbidities. Patients who were allocated to standard HCT with cyclophosphamide TBI conditioning, rather than the TN depletion trial, included patients who refused consent or lacked insurance coverage to participate in a clinical trial, those who were precluded from participating for logistical reasons, and those who did not meet eligibility criteria.
To ensure consistency in the trial, we established target ranges for the CD34+ cells, CD3+CD45RO–CD45RA+ TN, and total CD3+ T cells in the engineered stem cell grafts. A CD34+ cell dose of >5.0 × 106 cells/kg was selected because exceeding this threshold is associated with improved overall survival in recipients of PBSC HCT (31). A target of ≤7.5 × 104 TN/kg was chosen based on estimates that a quantity exceeding this number would be sufficient to cause GVHD. A target of 1 × 107 CD3+ T cells/kg, with an acceptable range of 1 × 106 to 10 × 106 CD3+ T cells/kg, was selected because this range of T cells is 10- to 100-fold greater than the number of unselected T cells predicted to cause GVHD after MRD HCT, and we reasoned that this number was likely to provide sufficient TM to facilitate immune reconstitution (32). We were successful in achieving these targets for all patients. As shown in Table 1, the PBSC grafts administered contained a median of 7.4 × 106 CD34+ cells/kg (range 5.1 × 106 to 19.9 × 106 CD34+ cells/kg) and 107 CD3+ T cells/kg that included 3,600 TN/kg (range 500–74,600 TN/kg; interquartile range 2,200–6,500 TN/kg). Eighty-six percent of patients received <10,000 TN/kg.
Donor cell engraftment. Successful and sustained engraftment of donor hematopoietic cells was a primary safety endpoint of the study. All patients had neutrophil engraftment, achieved at a median of 13 days (range 9–17 days), and platelet counts exceeded 20,000 per mm3 at a median of 14 days (range 9–111 days). Donor and recipient chimerism was determined by DNA genotyping for short tandem repeat polymorphisms. Myeloid (CD33+) engraftment was 100% donor in all recipients, and CD3+ T cells were 100% donor in most recipients after day 28 (Figure 2). There were no graft rejections, although one patient developed secondary graft failure at day 260 while still having 100% donor cells.
 Figure 2
Figure 2Engraftment and chimerism. Cumulative incidence of (A) neutrophil and (B) platelet engraftment. Percentage donor chimerism in sorted (C) CD33+ myeloid cells and (D) CD3+ T cells.
aGVHD. Twenty-three of the thirty-five patients developed clinical symptoms and signs consistent with grades II–IV aGVHD (66%; 95% CI 41%–74%), and the diagnosis was confirmed in all cases by biopsy of an involved site (Figure 3A). A test of the null hypothesis that the true rate of grades II–IV aGVHD is 60% yields P = 1.0 (1-sided binomial test), and therefore, we could not reject our prespecified null hypothesis for the second primary endpoint of the study. Three patients (9%; 95% CI 0%–18%) developed grade III aGVHD, and no cases of grade IV aGVHD or liver aGVHD were observed. The clinical pattern and stage of gastrointestinal (GI) and skin aGVHD are shown in Figure 3, B–D. Ten patients (29%) had no aGVHD; two (6%) developed grade I aGVHD (skin stage 2 only); thirteen (37%) had grade II GVHD limited to GI stage 1; seven (20%) had GI stage 1 and skin stage 1–2; and three (9%) had grade III aGVHD manifested by diarrhea (GI stage 2 [n = 1]; stage 3 [n = 2]) (Figure 3B).
 Figure 3
Figure 3GVHD. (A) Cumulative incidences of aGVHD by grade. (B) aGVHD organ involvement. Clinical severity of (C) GI aGVHD and (D) skin aGVHD. (E) Histopathological severity of GI GVHD on endoscopic biopsy samples. (F) Incidence of cGVHD.
All 23 patients with GI aGVHD developed symptoms while receiving prophylactic tacrolimus. Histologic grading of GVHD severity was performed in each case, and the scores were minimal in 9 patients and mild in 14 patients (Figure 3E). Twenty-two patients received systemic corticosteroids (median initial dose of 1 mg/kg; range 0.5–2 mg/kg) in addition to tacrolimus for treatment of grade II–IV aGVHD. Twenty patients had complete resolution of symptoms in <7 days, and the remaining 3 patients achieved a complete response in 7 to 14 days. Importantly, no patient required a second-line agent for treatment of aGVHD.
cGVHD. Three of the thirty-five TN-depleted recipients developed cGVHD, with a median follow-up of 932 days (range 209–1,826 days), for an estimated probability of 9% at 2 years (95% CI 0%–19%; Figure 3F). One patient had a skin rash and dry eyes (mild cGVHD); one patient had late upper GI GVHD, with mild oral changes (moderate cGVHD); and the third patient had upper GI GVHD, mild dry eyes, and developed deep sclerosis of the skin on the lower extremities during the taper of immunosuppression (severe cGVHD). The cGVHD in the latter patient resolved rapidly after initiation of rituximab, and systemic corticosteroids and rituximab were subsequently discontinued without cGVHD recurrence. All of the patients diagnosed with cGVHD had a preceding history of aGVHD. None of the patients diagnosed with grade III aGVHD subsequently developed cGVHD.
Adverse events and nonrelapse mortality. Adverse events were as expected for patients undergoing HCT following a myeloablative preparative regimen containing TBI (Supplemental Table 1; supplemental material available online with this article; doi:10.1172/JCI81229DS1). Nonrelapse mortality (NRM) occurred in 1 patient due to organ failure before day 100 and in 2 patients due to bacterial infections after day 100, resulting in 1- and 2-year estimates of 9% (95% CI 0%–19%; Figure 4A). No patient younger than 46 years old experienced NRM.
 Figure 4
Figure 4Survival, DFS, relapse, and NRM. The probabilities of (A) NRM, (B) overall survival and DFS, (C) relapse among all patients, and (D) relapse among patients in CR1, without minimal residual disease, or in CR2, CR3, and/or with residual disease.
Overall survival, disease-free survival, and relapse. Seven patients have died, and the 1- and 2-year estimates of overall survival were 82% (95% CI 65%–92%) and 78% (95% CI 59%–89%), respectively, with a median follow-up for the 28 survivors of 932 days (range 209–1,826 days). The disease-free survival (DFS) estimates at 1 and 2 years were 77% (95% CI 59%–88%) and 70% (95% CI 51%–83%), respectively (Figure 4B). The estimated probability of relapse was 14% (95% CI 3%–26%) at 1 year and 21% (95% CI 7%–35%) at 2 years (Figure 4C). Patients transplanted in first complete remission (CR1), without minimal residual disease (“better risk”) or with more advanced disease (beyond CR1 and/or with minimal residual disease; “poor risk”), had cumulative relapse incidences of 13% and 28% at 2 years, respectively (Figure 4D).
Infections. All documented infections occurring in the first 100 days were captured (Supplemental Table 2). EBV reactivation in blood was monitored weekly by PCR for the first 100 days in all patients, and we observed only a single low-level positive EBV PCR test result (41 copies/ml) in one patient. Seven patients developed mild self-resolving BK virus cystitis. CMV reactivation occurred in 19 patients, 54% of the total cohort and 73% of patients at risk based on recipient and/or donor CMV seropositivity.
Immune reconstitution. The recovery of blood lymphocyte subsets is shown in Figure 5. The median numbers of CD8+CD3+ T cells and CD4+CD3+ T cells were 177 per μl and 109 per μl at day 28, numbers which are comparable to those reported after T cell–replete MRD HCT and substantially higher than those reported after TCD HCT (Supplemental Table 3 and ref. 33). CD45RA+CD8+ and CD4+ TN were rarely observed before day 180, and Tregs remained infrequent throughout the first year after HCT (Figure 5). Concurrent with the appearance of TN, both TCR excision circles (TRECs) and TCR diversity, as measured by Vβ spectratyping, increased after day 180 (Figure 5H and Supplemental Figure 1).
 Figure 5
Figure 5Quantitative immune reconstitution. (A–G) Absolute numbers of (A) CD8+CD3+ T cells, (B) CD4+CD3+ T cells, (C) CD56+CD16+CD3– NK cells, (D) CD19+ B cells, (E) CD8+ TN, (F) CD4+ TN, and (G) Tregs. Error bars indicate the median value and interquartile range. The gray shading shows the normal range for each respective subset. H shows numbers of signal-joint TRECs (sjTRECs) per 106 PBMCs.
The recovery of T cells in the blood occurred well before emergence of thymic emigrants, consistent with a contribution from TM administered with the graft. We evaluated the transfer of virus-specific TM in a subset of 7 HLA-A*0201 CMV-seropositive TN-depleted HCT recipients with CMV-seropositive donors by measuring T cells in donor and recipient blood that were specific for a CMV pp65 peptide (pp65NLV) using an HLA A*0201/pp65NLV tetramer. By tetramer staining, T cells specific for pp65NLV were present in donor blood and in the blood of all 7 recipients 28 days after HCT (Figure 6, A and B). The pp65NLV-specific T cells contained CD27+CD28+ TCM and CD28– and/or CD27– TEM subsets and were functional, as demonstrated by an increase in cell frequency and number in temporal association with CMV reactivation and production of IFN-γ and IL-2 after pp65NLV peptide stimulation (Figure 6, A and C).
 Figure 6
Figure 6Transfer of CMV-specific T cells with TN-depleted HCT. (A and B) pp65NLV-specific T cells were detected by MHCI-tetramer staining of peripheral blood samples from HLA-A*0201+ CMV+ donors and in their respective recipients after HCT. (A) The time course of expansion of pp65NLV tetramer+ cells (right y axis, solid lines, absolute number of CD8+ pp65NLV+ T cells/μl in recipient peripheral blood at days 28, 56, and 80 after HCT) in relation to blood CMV copy number (left y axis, dashed lines) for 3 representative patients. Data shown at time 0 (black triangles) are from donor blood (D) (CD8+ pp65NLV+ T cells/μl). The 3 patients shown in A are representative of a total of 7 patients; the data for all 7 are shown in B. (B) The percentage of pp65NLV tetramer+ cells among CD8+ T cells and absolute numbers of pp65NLV tetramer+ CD8+ T cells in peripheral blood. (C) Expression of CD27 and CD28 on pp65NLV tetramer+ cells (blue overlay) and total CD8+ cells (red underlay) from 3 representative patients at day 56 and IL-2 and IFN-γ production on day 56 in response to pp65 peptide stimulation (gated on CD8+ cells).
Analysis of cGVHD in a contemporary T cell–replete cohort. The 9% incidence of cGVHD among TN-depleted HCT recipients is far lower than the rates of 40% to 63% reported for comparable patients transplanted with MRD T cell–replete HCT and ablative conditioning at our center and others (5–7). In a recent retrospective analysis of the frequency of cGVHD fulfilling NIH cGVHD consensus criteria and requiring systemic immunosuppression, a rate of 44% was found for MRD HCT recipients who received PBSCs between 1992 and 2008 (34). In order to exclude the possibility that the rate of cGVHD after MRD HCT had decreased over time independent of our intervention, we compared the incidence of GVHD in recipients of TN-depleted HCT with that in a cohort of patients that received T cell–replete HCT using a standard cyclophosphamide (120 mg/kg) and TBI (12 Gy) conditioning, with calcineurin inhibitor and methotrexate GVHD prophylaxis, during the same time period that our trial was conducted. The TN-depleted and T cell–replete groups were similar in age, disease type, and disease risk (Supplemental Table 4). The two groups did not differ in the frequency of grades II–IV aGVHD, sites of organ involvement (Figure 7A and Supplemental Figure 2, A and B), or aGVHD treatment. Steroid-refractory aGVHD is uncommon in HLA MRD transplantation, and, consistent with this, 0% and 3.1% of TN-depleted and T cell–replete recipients, respectively, developed steroid-refractory GVHD and required a second-line agent. Twenty of twenty-three TN-depleted HCT recipients and sixteen of twenty-two T cell–replete recipients who were treated for aGVHD with steroids responded within 7 days.
 Figure 7
Figure 7GVHD in recipients of TN-depleted PBSC HCT and the contemporary standard myeloablative PBSC HCT cohort. Cumulative incidences of (A) aGVHD and (B) cGVHD. Cumulative incidences of time to discontinuation of (C) systemic corticosteroids and of (D) all immunosuppression in recipients of TN-depleted and T cell–replete PBSC grafts.
Despite the similar incidences of aGVHD in TN-depleted and T cell–replete HCT recipients, cGVHD was far less frequent (9%; CI 0%–19%) after TN-depleted HCT compared with cGVHD after T cell–replete HCT (56%; CI 38%–73%) (Figure 7B). The median time to discontinuation of corticosteroids for GVHD treatment was 85 days in TN-depleted recipients, which was markedly shorter than the median of 853 days for the T cell–replete cohort (Figure 7C). Furthermore, the median time to discontinuation of all immunosuppression was 316 days among TN-depleted recipients and 1,478 days in the T cell–replete cohort (Figure 7D).
Male recipients of female grafts are at greater risk of cGVHD, and the TN-depleted cohort had fewer such recipients than the T cell–replete comparison group. In order to exclude the possibility that this disparity could explain the higher rate of cGVHD in T cell–replete HCT recipients, we analyzed GVHD in gender combinations other than male recipients of female grafts and found that cGVHD occurred in 11% (CI 0%–23%) of TN-depleted recipients and 48% (CI 27%–68%) of recipients of T cell–replete grafts. Thus, the apparent benefit of TN depletion for reducing cGVHD occurred independent of donor/recipient gender.
-
Discussion
This first-in-human trial demonstrates that engineering PBSC grafts to contain minimal numbers of TN while retaining CD34+ cells and TM is feasible. The 2-step graft-engineering strategy we developed to deplete TN was highly successful in achieving a profound depletion of TN (<7.5 × 104) relative to the usual dose of TN infused in PBSC HCT (1.8 × 108 TN CD3+/kg; ref. 29) and in meeting our target doses of CD34+ cells, TN, and total CD3+ T cells. Because 86% of patients received grafts containing <10,000 TN/kg, we were able to rigorously test the effect of TN depletion of the PBSC graft on outcome. Importantly, the trial met its primary safety endpoint to achieve durable donor hematopoietic engraftment, which occurred in 34 of 35 patients. The engraftment endpoint was important because pan-TCD has sometimes been associated with higher rates of graft failure and the engraftment potential of TN-depleted grafts had not been previously tested (9). Furthermore, the results in the first 35 patients treated with this approach to HCT provide the first experimental support to our knowledge for the hypothesis that transplanting allogeneic TM without TN will reduce cGVHD and preserve immune reconstitution, although it did not prevent aGVHD. The contrasting GVHD outcomes observed may initially appear unexpected but are actually consistent with our prior studies of transplantation of T cell subsets in murine models and are likely to reflect interesting and as yet incompletely understood aspects of GVHD biology in humans (17, 22, 23).
aGVHD was a second primary endpoint of the study and was not reduced by TN depletion. The observed rate of grade II–IV aGVHD of 66% was similar to the rates of grade II–IV aGVHD of 60% and 70% observed in unpublished historical and concurrent patients who received myeloablative allogeneic MRD HCT at our institution between 2001 and 2008 and 2008 and 2014, respectively. The rates of aGVHD are higher at FHCRC compared with other institutions, primarily due to the diagnosis of histologically mild aGVHD confined to the upper GI tract (35); hence, the importance of comparing our results to concurrent and historical aGVHD data from FHCRC. The frequency and severity of aGVHD after TN-depleted HCT was generally consistent with the pattern observed in recipients of T cell–replete MRD myeloablative HCT at our center, and upper GI aGVHD without skin or liver involvement was the predominant pattern of aGVHD in both the TN-depleted HCT recipients and T cell–replete comparison group. We observed no steroid-refractory aGVHD in TN-depleted HCT recipients (35).
Two factors in our study design could have contributed to a failure to detect a difference in aGVHD if TM are intrinsically less capable of causing aGVHD. First, historical and concurrent comparison patients received GVHD prophylaxis with a calcineurin inhibitor and a second agent, usually methotrexate, whereas patients in the TN depletion group received tacrolimus monotherapy. Second, the intensity of conditioning, the TBI dose in particular, is a risk factor for aGVHD (36, 37), and we used a conditioning regimen that is more intense than the cyclophosphamide/TBI regimen used in the comparison groups.
A notable finding in our study was the remarkably low rate (9%) of cGHVD, which is similar to the rate of 19% reported after CD34+-selected (TCD) HCT and lower than the rates of 40% to 63% reported after T cell–replete MRD HCT (5–7, 33) and of 56% in our concurrent comparison group. Although our study was single armed, it is unlikely that the cGVHD incidence was significantly underestimated. Patients undergo structured comprehensive long-term follow-up evaluations to screen for cGVHD by experts not associated with the study using standardized NIH criteria. The median time from HCT to the onset of cGVHD, as per the NIH criteria, after T cell–replete HCT is 162 days, and greater than 90% of cGVHD diagnoses are made in the first year after HCT (34). Furthermore, most patients who develop cGVHD do so while on pharmacological immunosuppression or within 3 months of its discontinuation. The patients in our trial have been followed for a median of 2.5 years, and 25 patients are beyond 1 year after HCT and have been off immunosuppression for many months in most cases. Patients diagnosed with cGVHD have an approximately 20% risk of NRM, inferior overall survival, and a greatly compromised quality of life (34, 38). The use of allogeneic PBSCs as a stem cell source has increased over the past decade and has remained high for logistical reasons, despite PBSCs being associated with a higher risk of cGVHD than bone marrow transplantation (5, 39–41). Because we observed a very low rate of cGVHD, even in the context of PBSC transplantation, our results suggest that TN depletion is likely to be a particularly relevant strategy for reducing the substantial cGVHD-related morbidity, mortality, and disability of allogeneic HCT.
It may seem surprising that we observed such a low rate of cGVHD in TN-depleted recipients, without a major effect on the occurrence of aGVHD. However, this is not inconsistent with our preclinical work. In mouse models, TEM consistently cause little to no GVHD, but we found that TCM could cause GVHD, albeit milder than TN, and the grafts infused in our clinical trial did contain both TEM and TCM (17, 22, 23). The pathogenesis of aGVHD and its relationship to cGVHD are not fully understood, and our clinical trial results suggest that the aGVHD syndrome that develops after TN-depleted HCT may differ biologically from that occurring after T cell–replete HCT, in that it less frequently presages the development of cGVHD. There are several possibilities that alone or in combination could account for biologic differences in GVHD after TN depletion that are the subject of ongoing work in our laboratories. The more limited TCR repertoire of TM could result in fewer minor H antigens being recognized by fewer T cell clones. The affinity of cross-reactive TM for minor H antigens may also be lower than that of alloreactive TN recruited into the response. TM progeny may also traffic differently, have a reduced ability to undergo sustained division, or have fewer pathogenic effector functions, including different cytokine profiles, as have been suggested in mouse studies (21, 25, 26). Thymic damage induced by aGVHD has been suggested to contribute to cGVHD pathogenesis through defective negative selection and impaired Treg development, and it is conceivable that TN depletion results in less thymus GVHD (42–47). An interesting hypothesis to explain the rarity of sustained alloreactivity after TN-depleted HCT is that the TM involved in the GVH syndrome do not primarily target minor H antigens but are instead directed against nonpolymorphic self antigens or microbial antigens. CD45RA+ Tregs are also removed by our graft manipulation, and transplantation of autologous Treg-depleted T cells in rodents induces GI inflammation, thought to be in part due to dysregulated immunity to microbial antigens (48–51). Finally, it is possible that the aGVHD syndrome observed in TN-depleted HCT recipients is primarily driven by cytokines such as IL-6 (52).
A rationale for our approach was that the transfer of TM would improve immune reconstitution relative to pan-TCD, which results in prolonged lymphopenia and an increase in opportunistic infections (10–12). T cell numbers recovered much earlier after TN-depleted HCT relative to patients that received TCD HCT (33), and EBV reactivation and post-HCT lymphoproliferative disease (PTLD), which occur in 18% and 2% of TCD HCT recipients, respectively (7, 33), were not observed at all after TN-depleted HCT, consistent with transfer of protective EBV-specific immunity. CMV reactivation is common after allogeneic HCT, independent of whether T cells are depleted or not, and the frequency of viral reactivation among patients at risk in the TN-depleted cohort (73%) and T cell–replete control group (87%) is similar to that reported in the literature for T cell–replete grafts (53, 54). Current practice dictates preemptive treatment of CMV reactivation at low levels of viremia after HCT, and progression to CMV disease is consequently now rare in HLA-matched HCT, precluding assessment of whether a particular HCT approach would change the natural history of CMV reactivation (55). However, we show that prior to the emergence of TRECs, CD8+ CMV-specific T cells were detected in the blood of TN-depleted HCT recipients and expanded in response to CMV reactivation, consistent with the transfer of functional anti-CMV immunity with the graft.
Within the limitations of a single-arm trial of this size, the reduction in cGVHD did not come with apparent decrements in other important clinical outcomes. The 2-year DFS rate of 70% in TN-depleted HCT recipients compares favorably to DFS rates of 50% to 65% after TCD or T cell–replete MRD HCT for acute leukemia (7, 33, 56, 57). Potentially fatal EBV reactivation and PTLD did not occur in TN-depleted recipients, and other serious infections were rare. Additionally, relapse was uncommon. In particular, the relapse rate of 28% observed among patients that received HCT with residual leukemia or a history of previous relapses compares favorably with the expected relapse rates of 37% to 60% with T cell–replete or TCD MRD HCT for this subset of patients, implying that the GVL effect may not be abrogated by TN depletion and/or that relapse is suppressed due to an earlier withdrawal of immunosuppressive drugs (7, 33, 57, 58).
Although we observed a large reduction in the rate of cGVHD relative to historical and concurrent patients that received T cell–replete HCT, this comparison of GVHD rates cannot substitute for a randomized trial. The TN-depleted and concurrent T cell–replete groups appear well balanced for known factors affecting HCT outcomes, but we cannot be sure that unrecognized risk factors were evenly distributed, so we did not conduct formal comparisons of survival. However, we were encouraged by the fact that there were no apparent increases in relapse or NRM among TN-depleted recipients. A prospective randomized controlled clinical trial will be required to prove that TN depletion reduces cGVHD without compromising immune reconstitution or increasing relapse rates compared with T cell–replete HCT and to determine whether a reduction in cGVHD results in meaningful improvements in survival, quality of life, or transplantation-related health care costs. Ideally, one would like to compare T cell–replete HCT, TN-depleted HCT, and T cell–depleted HCT in a single clinical trial. The trial could be designed such that there is a statistical comparison of the rates of aGVHD and cGVHD between T cell–replete and TN-depleted HCT and a descriptive prospective evaluation of immune reconstitution, CMV reactivation, and other opportunistic infections between TN-depleted and T cell–depleted HCT. It is likely that it will be feasible to use a uniform myeloablative conditioning, such as cyclophosphamide and TBI, for the T cell–replete and TN-depleted treatment arms, although the more intense TBI, fludarabine, and thiotepa conditioning would be required for recipients of T cell–depleted HCT.
If randomized trials confirm the substantial reduction in cGVHD with TN depletion observed in our trial, without impairment of other important HCT outcomes, this approach could be used at most HCT centers. During the period of patient accrual in our trial, 3 small pilot studies of TN depletion in recipients of HLA-mismatched or haploidentical HCT were initiated at other centers and published (59–61). Two of these studies administered antithymocyte globulin or alemtuzumab to provide additional TCD (59, 60), and the third study incorporated total lymphoid irradiation in the conditioning regimen and NK cell infusions after HCT (61). The small sizes of these studies; differences in the patient populations, donor sources, and conditioning; and the inclusion of T cell–depleting antibodies, total lymphoid irradiation, or additional cell infusions preclude comparisons of the reported patient outcomes with the results of our trial. However, these investigators achieved reproducible rigorous depletion of TN from the graft, confirming the reliability and general applicability of the new technology.
An important question is how TN depletion will compare to other approaches being developed for GVHD prevention. Single-arm studies of the administration of cyclophosphamide after HCT on days 3 and 4 after graft infusion to deplete activated alloreactive T cells in vivo have reported cGVHD rates of 13% and 31% for HLA-matched bone marrow and PBSC grafts, respectively, as well as grade II–IV aGVHD rates of 45% and 46% and 2- to 3-year DFS rates of 46% and 64% in patients with high-risk acute leukemia undergoing myeloablative HCT (62, 63). This strategy also does not appear to increase serious opportunistic infections, although a disadvantage is exposure of donor stem cells to high doses of an alkylating agent.
Despite the limitations of single-arm first-in-human clinical trials, such studies are pivotal for stimulating larger studies. The favorable DFS rate, preservation of functional T cell immunity, absence of steroid-refractory aGVHD, and, most compellingly, the very low rate of cGVHD with TN-depleted HCT warrant randomized controlled clinical trials of this approach in recipients of MRD grafts and extension to other transplant settings, including unrelated donor transplants, where severe aGVHD and cGVHD remain major obstacles to a successful outcome.
-
Methods
Graft engineering. The rationale and methodology for depleting TN using anti-CD45RA mAb-conjugated beads have been published previously (29). To allow precise T cell dosing and because a minor subset of CD34+ stem/progenitor cells express CD45RA, we used a 2-step immunomagnetic selection procedure involving positive selection of CD34+ progenitor cells, followed by depletion of CD45RA+ cells from the CD34-negative fraction (29). In brief, CD34+ selections were performed using the CliniMACS CD34 reagent system (Miltenyi Biotec) (33, 64), followed by depletion of CD45RA+ cells from the CD34– fraction using anti-CD45RA immunomagnetic beads (Miltenyi Biotec) (see Supplemental Methods for a list of reagents). The CD34-enriched and the CD45RA-depleted cell populations were each formulated in 100 ml Normosol-R (Hospira) with 1% human serum albumin prior to infusion.
Patients and treatment. Thirty-five patients, aged 19 to 55 years, with AML, ALL, or refractory anemia with excess blasts, who were candidates for myeloablative HCT and had a MRD, were enrolled on the phase II clinical trial at FHCRC (n = 33) or YUSM (n = 2) between December 2009 and July 2014. Eligible patients were considered by their referring physicians to require allogeneic HCT because they were judged to be at high risk of leukemic relapse following chemotherapy alone. Inclusion and exclusion criteria are detailed in the clinical trial protocol (Supplemental Methods). Patients at a very high risk of relapse after HCT due to a history of previous relapses or detectable disease immediately prior to HCT were designated “poor risk,” and those who had leukemia with high-risk cytogenetic or molecular characteristics but no prior history of relapse or detectable disease at the time of HCT were designated “better risk.” The conditioning regimen was composed of fludarabine (125 mg/m2), thiotepa (10 mg/kg), and TBI (1,320 cGy) (30).
Following the completion of conditioning, patients received a graft composed of CD34-selected PBSCs (≥5 × 106/kg) and CD45RA-depleted PBSCs containing a target dose of 107 CD3+ T cells/kg and ≤7.5 × 104 TN/kg. The cells were infused into the patient the same day as cell selection over 1 to 4 hours, with infusion of the CD34+-enriched cells followed immediately by infusion of the CD45RA-depleted cells. All patients received GVHD prophylaxis, with tacrolimus titrated to a serum level of 5 to 15 ng/ml and tapered after day 50 in the absence of GVHD or subsequently after GVHD resolution. We chose to use tacrolimus monotherapy, rather than a more intensive GVHD prophylaxis regimen, such as a combination of a calcineurin inhibitor with methotrexate or mycophenolate mofetil, because our intent was to evaluate TN depletion as an alternative rather than additional form of GVHD prophylaxis.
GVHD was treated according to institutional standard practice with systemic and/or topical corticosteroid administration and continuation of tacrolimus. Additional second-line GVHD therapies were permitted for the management of corticosteroid-resistant GVHD if necessary. The duration of full-dose systemic corticosteroids (0.5–2 mg/kg/d prednisone) and subsequent taper schedule were determined by the treating physician within the scope of institutional practice. Antimicrobial prophylaxis, infection definitions, monitoring and preemptive management, chimerism testing, and minimal residual disease evaluation are described in the Supplemental Methods. The clinical trial is registered at ClinicalTrials.gov (NCT 00914940). The full clinical trial protocol is available in the Supplemental Methods.
Endpoints. Primary endpoints were engraftment and the cumulative incidence of aGVHD grades II–IV. Predefined secondary endpoints were cGVHD, relapse, and day 100 NRM. An additional secondary objective was to evaluate immune reconstitution. Independent physician experts graded the peak stage of aGVHD and organ involvement and diagnosed and classified cGVHD according to the NIH consensus criteria (Supplemental Methods). Endoscopic GI and skin biopsies were performed to confirm GVHD and were graded by histological criteria (Supplemental Methods). Time to engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count of ≥500 cells per mm3. Donor chimerism was monitored by molecular techniques, and infections were defined and monitored as outlined (Supplemental Methods).
Contemporary comparison cohort. A cohort of patients that underwent HCT at FHCRC on a standard treatment plan during the same time period served as a comparison group for analysis of GVHD. This cohort represented all other patients aged 14 to 55 years who received TBI-containing myeloablative T cell–replete PBSC HCT from a HLA-MRD for the treatment of ALL, AML, or refractory anemia with excess blasts at FHCRC on a standard HCT treatment plan between April 2008 and March 2014. These patients received a standard conditioning regimen consisting of TBI (12 Gy) followed by cyclophosphamide (120 mg/kg) and GVHD prophylaxis, consisting of short-course methotrexate (15 mg/m2 day 1, 10 mg/m2 days 3, 6, and 11) and either tacrolimus (n = 28) or cyclosporine (n = 5). For inclusion in the comparison cohort, the patients had to meet the same age, disease status, and organ function eligibility criteria as patients treated in the TN-depleted PBSC clinical trial. The comparison group consisted primarily of patients who were unwilling or lacked insurance company approval to participate in an experimental clinical trial. Clinical trial patients and the comparison cohort received the same supportive care according to standard practice guidelines at FHCRC. Evaluations of clinical aGVHD and cGVHD were performed for TN-depleted clinical trial patients and the comparison cohort by the same expert evaluators who were not associated with the study. Histopathology of endoscopic GI and skin biopsies was assessed and graded by an expert GVHD pathologist in-house who was blinded to the transplant protocol.
Lymphocyte enumeration. Lymphocyte enumerations were performed by the Hematopathology Laboratory at the University of Washington using multicolor flow cytometry. Briefly, 100 μl of whole blood was labeled with mAbs, red blood cells were lysed (TQ-Prep; Beckman Coulter), and 10,000 mature lymphocyte events were acquired on an FC500 flow cytometer. TruCount beads (Becton Dickinson) were included and used to generate absolute counts for each population. The lymphocyte subsets were defined as follows using CXP software (Beckman Coulter): CD8+ T cells (CD8+CD3+), CD4+ T cells (CD4+CD3+), B cells (CD19+), and NK cells (CD3–CD56+ and/or CD16+). A separate 100-μl aliquot of sample was labeled with appropriately titered antibodies, red blood cells were lysed using NH4Cl containing 0.25% ultra-pure formaldehyde (Polysciences) and washed once with PBS-BSA, and up to 200,000 total events were acquired on a LSRII flow cytometer (Becton Dickinson). The additional lymphocyte subsets investigated using WoodList software were as follows: CD8+ TN (CD8+CD3+CD45RA+CD45RO–CD62L+), CD4+ TN (CD4+CD3+CD45RA+CD45RO–CD62L+), and Tregs (CD4+CD3+CD25+CD127–). CD127 expression has been demonstrated to correlate inversely with FOXP3 expression on CD4+CD25+ cells, and the CD4+CD25+CD127– phenotype is considered to be an acceptable surrogate marker for human Tregs and a practical alternative to intracellular staining for FOXP3 (65, 66). Antibodies were obtained from Beckman Coulter or Becton Dickinson. (See Supplemental Methods for a list of reagents).
TREC analysis. TREC analysis was performed in the FHCRC Immune Monitoring Shared Resource Facility. Signal-joint TRECs were evaluated in peripheral blood samples obtained on days 28, 56, 80, 180, and 360 after HCT. Primers and probes for TCR δ locus signal-joint TRECs were synthesized according to the methods described by Douek et al. (67). DNA was extracted from PBMCs and used as a template for real-time qPCR. To generate standard curves, plasmid DNA containing TREC and β-actin DNA segments was made into serial dilutions containing 102–106 copies per PCR reaction. Triplicate PCR reactions were run for each sample on the StepOnePlus real-time PCR system (Applied Biosystems).
Spectratyping. TCR spectratyping was performed in the FHCRC Immune Monitoring Shared Resource Facility. To assess TCR Vβ repertoire diversity in peripheral blood samples obtained at 6 and 12 months after HCT from TN-depleted HCT recipients, we used a multiplex PCR spectratyping method that amplifies 46 functional genes, comparing 23 TCRβV families in 5 reactions in which each reaction contains 4 to 7 specific primers, together with a single fluorescence-tagged TCR β constant region primer (68).
Antigen-specific T cell evaluation. MHC-tetramer analysis for CMV pp65 NLVPMVATV–specific (pp65NLV-specific) T cells was performed by flow cytometry using iTag MHC tetramers (Beckman Coulter) and mAbs specific for CD3, CD8, CD28, CD27, IFN-γ, and IL-2 (Becton Dickinson) (see Supplemental Methods for a list of reagents). Dead cell exclusion was performed using DAPI (Sigma-Aldrich) or Live/Dead Fixable Violet (Molecular Probes). PBMCs were surface labeled with antibodies and tetramers for 30 minutes at 4°C and evaluated on a LSRII flow cytometer. Analysis was performed using FlowJo software (Treestar). To assess function, aliquots of PBMCs were stimulated with pp65NLV peptide in the presence of anti-CD28 and anti-CD49a costimulatory mAbs (5 μl/ml; BD Biosciences). Brefeldin A (1 μl/ml Goligplug; BD Biosciences) was added 1.5 hours into the stimulation. After 6 hours, cells were stained with Live/Dead Fixable Violet, fixed, and permeabilized (Cytofix/Cytoperm, BD Biosciences) and then stained with fluorescent protein-conjugated mAbs against IFN-γ, IL-2, CD4, and CD8 (BD Biosciences) (see Supplemental Methods for a list of reagents) in Perm/Wash buffer (BD Biosciences), before washing and analysis on the flow cytometer.
Statistics. Data were analyzed as of December 2014. The protocol was designed with engraftment and grades II–IV aGVHD as the primary endpoints. We reviewed the FHCRC clinical research databases and derived estimates of the incidence of grade II–IV (60%) and III–IV (19%) aGVHD in patients undergoing HLA-MRD myeloablative HCT. Thirty-five patients provided 92% power to observe a statistically significant (1-sided significance level of 0.05) reduced probability of GVHD relative to the fixed rate of 60%, under the assumption that the true probability of grades II–IV GVHD is 35%. A 1-sided binomial test was performed in order to test the null hypothesis that the true rate of grade II–IV aGVHD is equal to the fixed rate of 60%. A P value of less than or equal to 0.05 was considered significant. Stopping rules were created such that the trial would stop prior to the accrual of 35 patients if the true probability of graft failure exceeded 5%. cGVHD was a predetermined secondary endpoint of the study. Probabilities of overall survival and DFS were estimated with the Kaplan-Meier method. Probabilities of death not preceded by relapse, recurrent malignancy, and GVHD were summarized with the use of cumulative incidence estimates, with recurrent malignancy viewed as a competing risk for death not preceded by relapse, with death not preceded by relapse viewed as a competing risk for recurrent malignancy, and with death without GVHD viewed as a competing risk for GVHD. Probabilities of discontinuation of systemic corticosteroids and of discontinuation of all system immune suppression were also summarized with cumulative incidence estimates, with death while still on corticosteroids or on any systemic immunosuppression viewed as a competing risk for discontinuation of corticosteroids or any systemic immune suppression, respectively. Statistical analyses of clinical outcomes were conducted using SAS 9.3 for Windows (SAS Institute).
Study approval. FHCRC and YUSM IRBs and the US FDA (Investigational Device Exemption 14160) approved the trial. Patients and donors provided written informed consent in accordance with the Declaration of Helsinki. A data safety monitoring board and an independent clinical trial monitor provided additional oversight. A concurrent cohort of patients undergoing HCT at FHCRC on a standard HCT treatment plan served as a comparison group for analysis of GVHD. The comparison group patients consented to review of their medical records and pathology.
-
Acknowledgments
The authors would like to thank Mary Flowers, Paul Martin, Brent Wood, Diane Krause, Taiga Nishihori, Lazarus Lekakis, Jan Storek, Steven Devine and the Blood and Marrow Transplant Clinical Trials Network 0303 study team, Andrew Mackie, Su Yi, Wendy Haskell, Julia Richardt, Sarah Click, Tanya Cunningham, Nick Culores, Jianhong Cao, Mason Lai, Tim Waters, Susan Lemmon, Amanda Peffer, Caroline McKallor, the Yale Clinical Trials Office staff, and members of the Data and Safety Monitoring Boards for their assistance. This work was supported by NIH grants CA18029, CA 136551, CA15704, HL121568-01A1, and DK56465 and by the NIH Rapid Access to Intervention Development (project 298). W. Shlomchik was the recipient of support from the Burroughs Wellcome Fund and was a Leukemia and Lymphoma Society Clinical Scholar. M. Bleakley is the Damon Runyon-Richard Lumsden Foundation Clinical Investigator, supported in part by the Damon Runyon Cancer Research and Richard Lumsden Foundations (CI-57-11), and received support from the National Cancer Institute (K23CA154532) and previously from a Special Fellowship in Clinical Research from the Leukemia and Lymphoma Society. Support for Blood and Marrow Transplant Clinical Trials Network 0303 (TCD) data shown in Supplemental Table 4 was provided by NIH (National Heart, Lung, and Blood Institute, National Cancer Institute) grant U10HL069294.
Address correspondence to: Marie Bleakley, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, Washington 98109, USA. Phone: 206.667.6572; E-mail: mbleakle@fredhutch.org.
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J Clin Invest. 2015;125(7):2677–2689. doi:10.1172/JCI81229.
-
References
- Pidala J, Djulbegovic B, Anasetti C, Kharfan-Dabaja M, Kumar A. Allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia (ALL) in first complete remission. Cochrane Database Syst Rev. 2011;(10):CD008818.
- Koreth J, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–2361.
- Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7(5):340–352.
- Gooley TA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101.
- Flowers ME, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100(2):415–419.
- Woolfrey A, et al. HLA-allele matched unrelated donors compared to HLA-matched sibling donors: role of cell source and disease risk category. Biol Blood Marrow Transplant. 2010;16(10):1382–1387.
- Pasquini MC, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30(26):3194–3201.
- Korngold R, Sprent J. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. Prevention by removing mature T cells from marrow. J Exp Med. 1978;148(6):1687–1698.
- Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98(12):3192–3204.
- Barker JN, et al. Serious infections after unrelated donor transplantation in 136 children: impact of stem cell source. Biol Blood Marrow Transplant. 2005;11(5):362–370.
- van Burik JA, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(12):1487–1498.
- Lee YJ, et al. Adenovirus viremia and disease: comparison of T cell-depleted and conventional hematopoietic stem cell transplantation recipients from a single institution. Biol Blood Marrow Transplant. 2013;19(3):387–392.View this article via: PubMed Google Scholar
- Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290(5489):92–97.
- Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286(5441):958–961.
- Robins HS, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114(19):4099–4107.
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500.
- Anderson BE, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112(1):101–108.
- Zhang Y, et al. Dendritic cell-activated CD44hiCD8+ T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood. 2004;103(10):3970–3978.
- Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L- memory T cells without graft-versus-host disease. Blood. 2004;103(4):1534–1541.View this article via: PubMed Google Scholar
- Dutt S, et al. Naive and memory T cells induce different types of graft-versus-host disease. J Immunol. 2007;179(10):6547–6554.View this article via: PubMed Google Scholar
- Chen BJ, et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007;109(7):3115–3123.View this article via: PubMed Google Scholar
- Zheng H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111(4):2476–2484.
- Zheng H, Matte-Martone C, Jain D, McNiff J, Shlomchik WD. Central memory CD8+ T cells induce graft-versus-host disease and mediate graft-versus-leukemia. J Immunol. 2009;182(10):5938–5948.
- Li N, et al. Memory T cells from minor histocompatibility antigen-vaccinated and virus-immune donors improve GVL and immune reconstitution. Blood. 2011;118(22):5965–5976.
- Zhang P, Wu J, Deoliveira D, Chao NJ, Chen BJ. Allospecific CD4(+) effector memory T cells do not induce graft-versus-host disease in mice. Biol Blood Marrow Transplant. 2012;18(10):1488–1499.
- Juchem KW, et al. A repertoire-independent and cell-intrinsic defect in murine GVHD induction by effector memory T cells. Blood. 2011;118(23):6209–6219.
- Anderson BE, et al. Enhancing alloreactivity does not restore GVHD induction but augments skin graft rejection by CD4(+) effector memory T cells. Eur J Immunol. 2011;41(9):2782–2792.
- Bleakley M, et al. Leukemia-associated minor histocompatibility antigen discovery using T-cell clones isolated by in vitro stimulation of naive CD8+ T cells. Blood. 2010;115(23):4923–4933.
- Bleakley M, et al. Engineering human peripheral blood stem cell grafts that are depleted of naive T cells and retain functional pathogen-specific memory T cells. Biol Blood Marrow Transplant. 2014;20(5):705–716.
- Jakubowski AA, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110(13):4552–4559.
- Collins NH, et al. The effect of the composition of unrelated donor bone marrow and peripheral blood progenitor cell grafts on transplantation outcomes. Biol Blood Marrow Transplant. 2010;16(2):253–262.
- Kernan NA, Collins NH, Juliano L, Cartagena T, Dupont B, O’Reilly RJ. Clonable T lymphocytes in T cell-depleted bone marrow transplants correlate with development of graft-v-host disease. Blood. 1986;68(3):770–773.View this article via: PubMed Google Scholar
- Devine SM, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17(9):1343–1351.View this article via: PubMed Google Scholar
- Flowers ME, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117(11):3214–3219.
- Martin PJ, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):320–327.
- Clift RA, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76(9):1867–1871.View this article via: PubMed Google Scholar
- Alyea E, et al. Effect of total body irradiation dose escalation on outcome following T-cell-depleted allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2002;8(3):139–144.
- Arai S, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118(15):4242–4249.
- Anasetti C, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496.View this article via: PubMed Google Scholar
- Bensinger WI, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344(3):175–181.View this article via: PubMed Google Scholar
- Mielcarek M, et al. Long-term outcomes after transplantation of HLA-identical related G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow. Blood. 2012;119(11):2675–2678.
- van den Brink MR, Moore E, Ferrara JL, Burakoff SJ. Graft-versus-host-disease-associated thymic damage results in the appearance of T cell clones with anti-host reactivity. Transplantation. 2000;69(3):446–449.
- Miura Y, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104(7):2187–2193.
- Zorn E, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903–2911.
- Zhang Y, Hexner E, Frank D, Emerson SG. CD4+ T cells generated de novo from donor hemopoietic stem cells mediate the evolution from acute to chronic graft-versus-host disease. J Immunol. 2007;179(5):3305–3314.
- Matsuoka K, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120(5):1479–1493.
- Wu T, et al. Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. J Immunol. 2013;191(1):488–499.
- Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5(11):1461–1471.View this article via: PubMed Google Scholar
- Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178(1):237–244.View this article via: PubMed Google Scholar
- Morrissey PJ, Charrier K. Induction of wasting disease in SCID mice by the transfer of normal CD4+/CD45RBhi T cells and the regulation of this autoreactivity by CD4+/CD45RBlo T cells. Res Immunol. 1994;145(5):357–362.
- Mayne CG, Williams CB. Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(8):1772–1788.View this article via: PubMed Google Scholar
- Kennedy GA, et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2014;15(13):1451–1459.
- Guerrero A, et al. Cytomegalovirus viral load and virus-specific immune reconstitution after peripheral blood stem cell versus bone marrow transplantation. Biol Blood Marrow Transplant. 2012;18(1):66–75.View this article via: PubMed Google Scholar
- Almyroudis NG, et al. Predictors for persistent cytomegalovirus reactivation after T-cell-depleted allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2007;9(4):286–294.
- Green ML, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1687–1699.
- Walter RB, et al. Comparison of matched unrelated and matched related donor myeloablative hematopoietic cell transplantation for adults with acute myeloid leukemia in first remission. Leukemia. 2010;24(7):1276–1282.View this article via: PubMed Google Scholar
- Doney K, Gooley TA, Deeg HJ, Flowers ME, Storb R, Appelbaum FR. Allogeneic hematopoietic cell transplantation with full-intensity conditioning for adult acute lymphoblastic leukemia: results from a single center, 1998–2006. Biol Blood Marrow Transplant. 2011;17(8):1187–1195.
- Walter RB, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122(10):1813–1821.
- Touzot F, et al. CD45RA depletion in HLA-mismatched allogeneic hematopoietic stem cell transplantation for primary combined immunodeficiency: a preliminary study. J Allergy Clin Immunol. 2015;135(5):1303–1309.e3.
- Shook DR, Triplett BM, Eldridge PW, Kang G, Srinivasan A, Leung W. Haploidentical stem cell transplantation augmented by CD45RA negative lymphocytes provides rapid engraftment and excellent tolerability. Pediatr Blood Cancer. 2015;62(4):666–673.
- Triplett BM, et al. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. [published online ahead of print February 9, 2015]. Bone Marrow Transplant. doi:10.1038/bmt.2014.324.
- Kanakry CG, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124(25):3817–3827.
- Solomon SR, et al. Calcineurin inhibitor — free graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide and brief-course sirolimus following reduced-intensity peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(11):1828–1834.View this article via: PubMed Google Scholar
- Keever-Taylor CA, et al. Characteristics of CliniMACS(R) System CD34-enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia-Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303. Biol Blood Marrow Transplant. 2012;18(5):690–697.View this article via: PubMed Google Scholar
- Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–1711.
- Seddiki N, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693–1700.View this article via: PubMed Google Scholar
- Douek DC, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355(9218):1875–1881.
- Akatsuka Y, Martin EG, Madonik A, Barsoukov AA, Hansen JA. Rapid screening of T-cell receptor (TCR) variable gene usage by multiplex PCR: application for assessment of clonal composition. Tissue Antigens. 1999;53(2):122–134.
-
Version history
- Version 1 (June 8, 2015): No description
- Version 2 (July 1, 2015): No description



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)