Citation Information: J Clin Invest. 2004;114(9):1241-1244. https://doi.org/10.1172/JCI23467.
Abstract
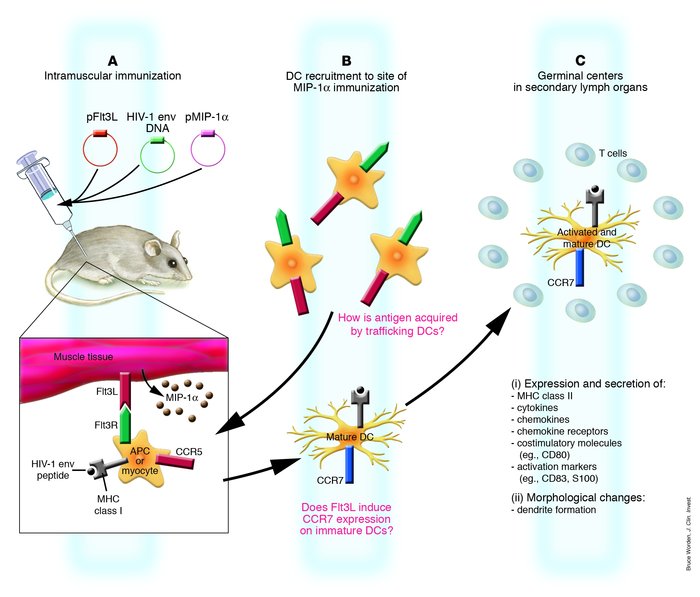
DNA vaccination is a novel immunization strategy that has great potential for the development of vaccines and immune therapeutics. This strategy has been highly effective in mice, while less immunogenic in nonhuman primates and humans. Enhancing DNA vaccine potency remains a challenge. It is likely that APCs, and especially DCs, play a paramount role in the presentation of vaccine antigen to the immune system. A new study reports the synergistic recruitment, expansion, and activation of DCs in vivo in a mouse model through covaccination with plasmids encoding macrophage inflammatory protein-1α (MIP-1α), fms-like tyrosine kinase 3 ligand (Flt3L), and the DNA vaccine. Such cooperative strategies delivering vaccine in a single, simple platform result in improved cellular immunity in vivo, including enhanced tetramer responses and IFN-γ secretion by antigen-specific cells.
Authors
Michele A. Kutzler, David B. Weiner
Figure 1



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)