Advertisement
ArticleHematology Free access | 10.1172/JCI21982
Loss of α-hemoglobin–stabilizing protein impairs erythropoiesis and exacerbates β-thalassemia
Yi Kong,1 Suiping Zhou,2 Anthony J. Kihm,2 Anne M. Katein,3 Xiang Yu,1 David A. Gell,4 Joel P. Mackay,4 Kazuhiko Adachi,2 Linda Foster-Brown,3 Calvert S. Louden,3 Andrew J. Gow,2 and Mitchell J. Weiss2
1Cell and Molecular Biology Graduate Program, The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 2The Children’s Hospital of Philadelphia and The University of Pennsylvania, Philadelphia, Pennsylvania, USA. 3Safety Assessment, Astra-Zeneca Pharmaceuticals, L.P., Wilmington, Delaware, USA. 4School of Molecular and Microbial Biosciences, University of Sydney, New South Wales, Australia.
Address correspondence to: Mitchell J. Weiss, Children’s Hospital of Philadelphia and The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104, USA. Phone: (215) 590-0565; Fax: (215) 590-4834; E-mail: weissmi@email.chop.edu.
Find articles by Kong, Y. in: JCI | PubMed | Google Scholar
1Cell and Molecular Biology Graduate Program, The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 2The Children’s Hospital of Philadelphia and The University of Pennsylvania, Philadelphia, Pennsylvania, USA. 3Safety Assessment, Astra-Zeneca Pharmaceuticals, L.P., Wilmington, Delaware, USA. 4School of Molecular and Microbial Biosciences, University of Sydney, New South Wales, Australia.
Address correspondence to: Mitchell J. Weiss, Children’s Hospital of Philadelphia and The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104, USA. Phone: (215) 590-0565; Fax: (215) 590-4834; E-mail: weissmi@email.chop.edu.
Find articles by Zhou, S. in: JCI | PubMed | Google Scholar
1Cell and Molecular Biology Graduate Program, The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 2The Children’s Hospital of Philadelphia and The University of Pennsylvania, Philadelphia, Pennsylvania, USA. 3Safety Assessment, Astra-Zeneca Pharmaceuticals, L.P., Wilmington, Delaware, USA. 4School of Molecular and Microbial Biosciences, University of Sydney, New South Wales, Australia.
Address correspondence to: Mitchell J. Weiss, Children’s Hospital of Philadelphia and The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104, USA. Phone: (215) 590-0565; Fax: (215) 590-4834; E-mail: weissmi@email.chop.edu.
Find articles by Kihm, A. in: JCI | PubMed | Google Scholar
1Cell and Molecular Biology Graduate Program, The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 2The Children’s Hospital of Philadelphia and The University of Pennsylvania, Philadelphia, Pennsylvania, USA. 3Safety Assessment, Astra-Zeneca Pharmaceuticals, L.P., Wilmington, Delaware, USA. 4School of Molecular and Microbial Biosciences, University of Sydney, New South Wales, Australia.
Address correspondence to: Mitchell J. Weiss, Children’s Hospital of Philadelphia and The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104, USA. Phone: (215) 590-0565; Fax: (215) 590-4834; E-mail: weissmi@email.chop.edu.
Find articles by Katein, A. in: JCI | PubMed | Google Scholar
1Cell and Molecular Biology Graduate Program, The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 2The Children’s Hospital of Philadelphia and The University of Pennsylvania, Philadelphia, Pennsylvania, USA. 3Safety Assessment, Astra-Zeneca Pharmaceuticals, L.P., Wilmington, Delaware, USA. 4School of Molecular and Microbial Biosciences, University of Sydney, New South Wales, Australia.
Address correspondence to: Mitchell J. Weiss, Children’s Hospital of Philadelphia and The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104, USA. Phone: (215) 590-0565; Fax: (215) 590-4834; E-mail: weissmi@email.chop.edu.
Find articles by Yu, X. in: JCI | PubMed | Google Scholar
1Cell and Molecular Biology Graduate Program, The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 2The Children’s Hospital of Philadelphia and The University of Pennsylvania, Philadelphia, Pennsylvania, USA. 3Safety Assessment, Astra-Zeneca Pharmaceuticals, L.P., Wilmington, Delaware, USA. 4School of Molecular and Microbial Biosciences, University of Sydney, New South Wales, Australia.
Address correspondence to: Mitchell J. Weiss, Children’s Hospital of Philadelphia and The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104, USA. Phone: (215) 590-0565; Fax: (215) 590-4834; E-mail: weissmi@email.chop.edu.
Find articles by Gell, D. in: JCI | PubMed | Google Scholar
1Cell and Molecular Biology Graduate Program, The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 2The Children’s Hospital of Philadelphia and The University of Pennsylvania, Philadelphia, Pennsylvania, USA. 3Safety Assessment, Astra-Zeneca Pharmaceuticals, L.P., Wilmington, Delaware, USA. 4School of Molecular and Microbial Biosciences, University of Sydney, New South Wales, Australia.
Address correspondence to: Mitchell J. Weiss, Children’s Hospital of Philadelphia and The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104, USA. Phone: (215) 590-0565; Fax: (215) 590-4834; E-mail: weissmi@email.chop.edu.
Find articles by Mackay, J. in: JCI | PubMed | Google Scholar
1Cell and Molecular Biology Graduate Program, The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 2The Children’s Hospital of Philadelphia and The University of Pennsylvania, Philadelphia, Pennsylvania, USA. 3Safety Assessment, Astra-Zeneca Pharmaceuticals, L.P., Wilmington, Delaware, USA. 4School of Molecular and Microbial Biosciences, University of Sydney, New South Wales, Australia.
Address correspondence to: Mitchell J. Weiss, Children’s Hospital of Philadelphia and The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104, USA. Phone: (215) 590-0565; Fax: (215) 590-4834; E-mail: weissmi@email.chop.edu.
Find articles by Adachi, K. in: JCI | PubMed | Google Scholar
1Cell and Molecular Biology Graduate Program, The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 2The Children’s Hospital of Philadelphia and The University of Pennsylvania, Philadelphia, Pennsylvania, USA. 3Safety Assessment, Astra-Zeneca Pharmaceuticals, L.P., Wilmington, Delaware, USA. 4School of Molecular and Microbial Biosciences, University of Sydney, New South Wales, Australia.
Address correspondence to: Mitchell J. Weiss, Children’s Hospital of Philadelphia and The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104, USA. Phone: (215) 590-0565; Fax: (215) 590-4834; E-mail: weissmi@email.chop.edu.
Find articles by Foster-Brown, L. in: JCI | PubMed | Google Scholar
1Cell and Molecular Biology Graduate Program, The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 2The Children’s Hospital of Philadelphia and The University of Pennsylvania, Philadelphia, Pennsylvania, USA. 3Safety Assessment, Astra-Zeneca Pharmaceuticals, L.P., Wilmington, Delaware, USA. 4School of Molecular and Microbial Biosciences, University of Sydney, New South Wales, Australia.
Address correspondence to: Mitchell J. Weiss, Children’s Hospital of Philadelphia and The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104, USA. Phone: (215) 590-0565; Fax: (215) 590-4834; E-mail: weissmi@email.chop.edu.
Find articles by Louden, C. in: JCI | PubMed | Google Scholar
1Cell and Molecular Biology Graduate Program, The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 2The Children’s Hospital of Philadelphia and The University of Pennsylvania, Philadelphia, Pennsylvania, USA. 3Safety Assessment, Astra-Zeneca Pharmaceuticals, L.P., Wilmington, Delaware, USA. 4School of Molecular and Microbial Biosciences, University of Sydney, New South Wales, Australia.
Address correspondence to: Mitchell J. Weiss, Children’s Hospital of Philadelphia and The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104, USA. Phone: (215) 590-0565; Fax: (215) 590-4834; E-mail: weissmi@email.chop.edu.
Find articles by Gow, A. in: JCI | PubMed | Google Scholar
1Cell and Molecular Biology Graduate Program, The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 2The Children’s Hospital of Philadelphia and The University of Pennsylvania, Philadelphia, Pennsylvania, USA. 3Safety Assessment, Astra-Zeneca Pharmaceuticals, L.P., Wilmington, Delaware, USA. 4School of Molecular and Microbial Biosciences, University of Sydney, New South Wales, Australia.
Address correspondence to: Mitchell J. Weiss, Children’s Hospital of Philadelphia and The University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104, USA. Phone: (215) 590-0565; Fax: (215) 590-4834; E-mail: weissmi@email.chop.edu.
Find articles by Weiss, M. in: JCI | PubMed | Google Scholar
Published November 15, 2004 - More info
J Clin Invest. 2004;114(10):1457–1466. https://doi.org/10.1172/JCI21982.
© 2004 The American Society for Clinical Investigation
Received: April 27, 2004; Accepted: September 14, 2004
-
Abstract
Hemoglobin (Hb) A production during red blood cell development is coordinated to minimize the deleterious effects of free α- and β-Hb subunits, which are unstable and cytotoxic. The α-Hb–stabilizing protein (AHSP) is an erythroid protein that specifically binds α-Hb and prevents its precipitation in vitro, which suggests that it may function to limit free α-Hb toxicities in vivo. We investigated this possibility through gene ablation and biochemical studies. AHSP–/– erythrocytes contained hemoglobin precipitates and were short-lived. In hematopoietic tissues, erythroid precursors were elevated in number but exhibited increased apoptosis. Consistent with unstable α-Hb, AHSP–/– erythrocytes contained increased ROS and evidence of oxidative damage. Moreover, purified recombinant AHSP inhibited ROS production by α-Hb in solution. Finally, loss of AHSP worsened the phenotype of β-thalassemia, a common inherited anemia characterized by excess free α-Hb. Together, the data support a model in which AHSP binds α-Hb transiently to stabilize its conformation and render it biochemically inert prior to Hb A assembly. This function is essential for normal erythropoiesis and, to a greater extent, in β-thalassemia. Our findings raise the possibility that altered AHSP expression levels could modulate the severity of β-thalassemia in humans.
-
Introduction
Late-stage erythroid development is largely dedicated to the production of the oxygen carrier hemoglobin (Hb) A, a tetramer consisting of two pairs of α-globin and β-globin protein subunits with each monomer bound to a heme moiety. Hb A synthesis is exquisitely coordinated to minimize the accumulation of free α- or β-Hb subunits, which are cytotoxic. Excessive α-Hb is particularly damaging, as evidenced by β-thalassemia, a common inherited anemia in which mutations in the β-globin gene impair the production of β-Hb with consequent buildup of the unpaired α-subunit (1–5). Intact monomeric α-Hb generates ROS that damage cellular proteins, lipids, and nucleic acids (6). In addition, α-Hb is structurally unstable, with a tendency to denature upon oxidation, filling the cytoplasm and cell membrane with precipitated α-globin polypeptides, free heme, porphyrins, and iron, which further propagate ROS production (reviewed in ref. 7). Together, these effects reduce the lifespan of circulating erythrocytes and also impair the viability of erythroid precursors in hematopoietic tissues, causing ineffective erythropoiesis.
Most cells contain compensatory mechanisms to cope with unstable proteins (8). These include molecular chaperones that stabilize proteins and in some cases facilitate their folding into native structures. In addition, there are degradation pathways that recognize and eliminate improperly folded polypeptides. Accordingly, tissues typically tolerate some protein instability, with disease ensuing only when the compensatory mechanisms become overwhelmed. Several findings indicate that mechanisms to neutralize free α-Hb exist in erythroid cells. First, erythroid precursors contain a small pool of excess free α-Hb with no apparent ill effects (9, 10). In addition, erythropoiesis is typically relatively normal in humans lacking one functional β-globin gene (β-thalassemia trait). Finally, there is frequent unexplained phenotypic diversity among individuals with the same β-thalassemia genotype (reviewed in ref. 11). The last observation could be explained by genetic variations in processes that stabilize or eliminate free α-Hb. Mechanisms to degrade excess free α-Hb in thalassemic erythroid cells were first recognized by Bank and O’Donnell in 1969 (12) and were later shown to be mediated through ubiquitin-dependent proteolytic pathways by Shaeffer and colleagues (13–15). More recently, we identified α-Hb–stabilizing protein (AHSP), also known as erythroid-associated factor (ERAF), a candidate molecular chaperone for α-Hb (16, 17).
AHSP was identified as an erythroid-specific protein whose gene was induced by the essential transcription factor GATA-1 (16, 17). AHSP heterodimerizes with α-Hb (Kd, approximately 100 nM), but does not bind β-Hb or Hb A. Moreover, α-Hb bound to AHSP is more resistant to oxidant-induced precipitation than α-Hb alone. Based on these findings, we hypothesized that AHSP might protect erythroid cells from α-Hb toxicity by maintaining α-Hb in a stable state prior to its incorporation into Hb A. To test this, we generated AHSP–/– mice by gene targeting. Preliminary analysis of these animals revealed abnormal erythrocyte morphology with hemoglobin precipitates (Heinz bodies) (17). Here we have examined these mutant mice in greater detail to gain further insights into the molecular actions of AHSP in vivo. We found that loss of AHSP reduced the lifespan of circulating red blood cells and also caused increased apoptosis of erythroid precursors. These effects were mediated, at least in part, by increased production of ROS with consequent damage to Hb A and other cellular components. Moreover, AHSP blocked ROS production by α-Hb directly. Finally, through interbreeding of mutant mice, we show that loss of AHSP worsened the severity of β-thalassemia. Together, these findings indicate that AHSP acts as protein-specific molecular chaperone that detoxifies free α-Hb during normal erythropoiesis and in pathological states of α-Hb excess.
-
Results
Loss of AHSP causes hemolytic anemia with globin chain precipitation. To study the hematopoietic consequences of AHSP loss, we disrupted the gene in mice as described previously (17). Our targeting strategy replaced the entire protein-encoding region with a phosphoglycerate kinase promoter–neomycin-resistance gene (PGK-NeoR) cassette flanked by loxP recombination sites. The hematopoietic abnormalities in AHSP–/– animals reported below were identical when the PGK-NeoR expression cassette was either present or removed by Cre-mediated recombination (not shown).
AHSP–/– and AHSP+/– mice were born at the expected mendelian ratios and displayed no gross abnormalities compared with wild-type (AHSP+/+) littermates. AHSP–/– mice exhibited normal lifespans up to at least 18 months of age. As expected, there was no AHSP RNA or AHSP protein detected in hematopoietic tissues of AHSP–/– animals (17). Hematological analysis of the gene-targeted mice performed using an automated analyzer (Bayer ADVIA 120) revealed several new findings not appreciated previously (Table 1). No abnormalities in platelets or white blood cells were detected, but there were several obvious erythroid defects. AHSP–/– animals exhibited a mild but significant anemia associated with small red blood cells (low mean corpuscular volume [MCV]) containing decreased Hb (low mean corpuscular Hb [MCH]). There was significant variation in the size and hemoglobin content of the mutant erythrocytes, as evidenced by increased rbc distribution width (RDW) and Hb distribution width (HDW), respectively. The reticulocyte count was elevated in a subset of AHSP–/– mice, indicating increased erythrocyte production to compensate for accelerated loss or destruction.
The blood smears of AHSP–/– mice showed numerous morphologic abnormalities, including irregular size and shape, target cells, and spiculated cells (Figure 1A, upper right panel). There were increased polychromatophilic cells representing newly synthesized erythrocytes; many of these contained eosinophilic inclusions. Inclusion bodies were also apparent in AHSP–/– erythrocytes after staining with crystal violet, which detects denatured globin chains (Heinz bodies, Figure 1A, lower panels). Of note, AHSP+/– erythrocytes also contained occasional Heinz bodies, suggesting haploinsufficiency effects (see below). Unstable denatured Hbs can accelerate erythrocyte destruction by causing intravascular hemolysis and/or sequestration by the reticuloendothelial system. In support of this, the presence of large inclusions only in polychromatophilic cells suggests that these are rapidly cleared from the circulation. This preferential loss of nascent erythroid cells could result in a reticulocyte count that is lower than expected for the degree of erythrocyte destruction.
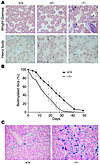 Figure 1
Figure 1AHSP–/– erythrocytes exhibit abnormal morphology, hemoglobin precipitates (Heinz bodies), and reduced lifespan. (A) Wright-Giemsa staining (upper panels) shows eosinophilic inclusion bodies (*) in AHSP–/– erythrocytes. Heinz body staining, which detects denatured globin chains (lower panels), is weakly positive in some AHSP+/– erythrocytes (indicated by carets) and is strongly positive in AHSP–/– cells. The boxed area in the top right panel shows an enlargement of an inclusion body. Original magnification, ∞1,000. (B) Erythrocyte survival kinetics determined by biotin labeling. Circulating erythrocytes in 5 animals of each genotype were biotinylated at days –2 and –1. Beginning at day 0, approximately 5 ∝l of blood was removed from the tail vein at the indicated time points, and the fraction of biotin-labeled erythrocytes was quantified by flow cytometry. The half-life of wild-type red blood cells was 22 days, whereas that of the AHSP–/– red blood cells was 12 days. AHSP+/– erythrocytes exhibited normal survival kinetics (not shown). (C) Prussian blue staining for cellular iron in spleen. Increased iron in the AHSP–/– spleen reflects accelerated clearance of erythroid cells by the reticuloendothelial system. Original magnification, ∞200.
To determine the effects of AHSP loss on erythrocyte lifespan more directly, we injected animals with N-hydroxysuccinimide–biotin (NHS-biotin), which labeled nearly all circulating red blood cells with biotin (Figure 1B). The survival of tagged erythrocytes was then monitored over a 50-day period by removal of small samples (approximately 5 μl) of blood from the tail vein, staining with FITC-streptavidin, and quantification of the fraction of labeled cells using flow cytometry. Erythrocytes from normal littermates exhibited a half-life of about 22 days, in accordance with previous studies (18). In contrast, the half-life of AHSP–/– erythrocytes was significantly shortened to about 12 days. Hence, loss of AHSP causes significant premature destruction of circulating red blood cells. Histological examination of spleens from AHSP–/– mice showed engulfment of erythroid cells by macrophage (erythrophagocytosis; not shown) and increased macrophage iron, as detected by Prussian blue staining (Figure 1C). These findings are consistent with accelerated removal of mutant erythrocytes and/or erythroid precursors by the reticuloendothelial system (also see below).
Hyperplasia and excessive apoptosis of AHSP–/– erythroid precursors. In most disorders of erythrocyte destruction, there is a compensatory increase in production of erythroid precursors in hematopoietic tissues. The spleen, a major site of erythropoiesis in mice, was significantly enlarged in AHSP–/– mice (Figure 2A). Flow cytometry showed an increased proportion of erythroid precursors in AHSP–/– spleens, as measured by expression of the late-stage erythroid-specific cell surface marker Ter119 (Figure 2B). To further examine erythroid precursors, we disaggregated spleens into single-cell suspensions and seeded them into semisolid medium. This assay assesses the developmental potential of individual cells and identifies 2 types of committed erythroid precursors: burst-forming unit erythroid (BFUe) cells represent early-stage precursors that give rise to large hemoglobinized colonies in the presence of erythropoietin (Epo) and stem cell factor (SCF); CFU erythroid (CFUe) precursors represent a later stage of development and give rise to smaller colonies in the presence of Epo alone. As shown in Figure 2C, the proportions of both BFUe and CFUe precursors were elevated in AHSP–/– mice. In contrast, myeloid progenitors, which give rise to granulocyte- and macrophage-containing colonies in appropriate cytokines, were unchanged. Finally, histological analysis showed notable expansion of the splenic red pulp in the knockout animals (not shown). Therefore, splenic enlargement observed in AHSP–/– mice is due to expansion of the erythroid compartment. We also detected significant erythroid hyperplasia in the bone marrow of AHSP–/– mice, although to a lesser extent than observed in the spleen (not shown). These results reflect increased erythropoietic drive to compensate for accelerated destruction of mature AHSP–/– erythrocytes.
 Figure 2
Figure 2Erythroid hyperplasia in AHSP–/– mice. (A) Increased spleen weight in AHSP–/– mice. (B) Elevated proportion of Ter119+ erythroid cells in AHSP–/– mice, as determined by flow cytometry. (C) Methylcellulose progenitor assays.*P < 0.005. n = 6 for each genotype.
In β-thalassemia, accumulation of excess free α-Hb not only damages mature erythrocytes but also induces apoptosis of erythroid precursors in hematopoietic tissues in a process called ineffective erythropoiesis (1). To investigate whether loss of AHSP causes ineffective erythropoiesis, we examined splenocytes for coexpression of Ter119 and the transferrin receptor CD71, which allows subdivision of erythroid precursors according to their maturation stage (Figure 3A) (19). AHSP–/– mice exhibited an elevated proportion of immature erythroid precursors (Ter119 high, CD71 high) compared with that of mature ones (Ter119 high, CD71 low), suggesting that the transition from immature to mature precursor was partially blocked (Figure 3, A and B). This effect could reflect ineffective erythropoiesis, which is characterized by premature death of erythroid precursors in hematopoietic tissues. We assessed this by direct immunohistochemical examination (Figure 3, C–F). Erythroid cells were identified by expression of Ter119 (brown staining in Figure 3, C and D). Apoptotic cells were identified by TUNEL staining, which detects endonucleolytic cleavage of nuclear DNA (red staining in Figure 3, C–E). AHSP–/– spleens contained an increased proportion of Ter119-staining cells, consistent with the flow cytometry data in Figure 2B, above. In addition, apoptosis of erythroid precursors was significantly elevated in the mutant animals, as evidenced by an increased proportion of Ter119+ cells that were also TUNEL+ (Figure 3F). Hence, loss of AHSP not only damages mature erythrocytes, as demonstrated above, but also is toxic to erythroid precursors, similar to the effects of excess α-Hb in β-thalassemia and consistent with a role for AHSP in stabilizing free α-Hb.
 Figure 3
Figure 3Ineffective erythropoiesis in AHSP–/– mice. (A) Analysis of splenic erythroid precursors according to levels of Ter119 and CD71 expression. Regions a through d (boxed areas) represent increasingly mature stages of erythroid development (19). APC, allophycocyanin. PE, phycoerythrin. (B) Ratio of mature to immature erythroid precursors in spleen calculated according to levels of Ter119 and CD71 expression defined by flow cytometry gates in panel A: Percent mature cells = number of cells in d / (a + b + c + d); n = 6 animals for each genotype; **P < 0.05. (C–E) Detection of apoptosis in erythroid precursors. Spleen sections were stained for the erythroid surface marker Ter119 (brown) and for nuclear endonucleolytic cleavage using the TUNEL assay (red). Examples of normal viable erythroid precursors (Ter119+TUNEL–) are indicated by arrows. Examples of apoptotic erythroid precursors (Ter119+TUNEL+) are indicated by arrowheads. AHSP genotypes are indicated. E is an enlargement of the boxed region in D. Original magnification in C–E, ×400. (F) Percent apoptotic erythroid precursors in AHSP–/– mice and wild-type littermates (n = 4 mice of each genotype).
Loss of AHSP results in bothα- and β-globin precipitates. Next, we characterized the globin precipitates in AHSP–/– mice. In disorders associated with unstable hemoglobins, including thalassemias, denatured globins precipitate onto the cell membrane and damage associated lipids and proteins (20–27). We isolated plasma membranes from erythrocytes of mutant mice and wild-type littermates, extracted them with detergent to remove lipids, and analyzed the resultant membrane skeletons for associated globins by Triton–acetic acid–urea (TAU) gel electrophoresis (Figure 4A). Although we never observed erythrocyte membrane–associated globin chains in control littermates, about half of the AHSP+/– (heterozygous) mice examined showed low-level α-globin precipitate, consistent with the presence of occasional Heinz bodies noted in Figure 1A, above. These findings indicate that AHSP haploinsufficiency destabilizes α-Hb to some extent but that this does not exceed a threshold level required for clinically significant erythroid pathology.
 Figure 4
Figure 4Unstable hemoglobins in AHSP–/– erythrocytes. (A) TAU gel analysis of membrane-associated globin chains with the AHSP genotypes indicated at the top. Each lane represents membrane skeletons prepared from the same number of erythrocytes. Right margin: α, α-globin; β, β-globin. (B) Isopropanol hemoglobin stability test. Fresh hemolysates were incubated with isopropanol (17% volume/volume) at 37°C, and hemoglobin precipitation was quantified at the indicated times. AHSP genotypes are indicated. The hemoglobin stabilities were significantly different at all time points for the two genotypes (n = 5 animals of each genotype; P < 0.01).
Surprisingly, AHSP–/– erythrocytes contained both α- and β-globin precipitates at roughly equimolar ratios, despite our previous findings that AHSP specifically binds and stabilizes α-Hb. One explanation for this apparent paradox is that excess free α-Hb can produce ROS that oxidize and destabilize Hb A (28–30), eventually causing it to precipitate. In this case, unstable Hb A should be present in AHSP–/– erythrocytes. To test this, we prepared soluble Hb A from fresh hemolysates and examined its stability after the addition of 17% isopropanol, which causes preferential precipitation of unstable hemoglobins (31). As illustrated in Figure 4B, soluble Hb A from AHSP–/– erythrocytes precipitated more readily in isopropanol, suggesting prior damage. Taken together with our prior biochemical data, these findings indicate that loss of AHSP destabilizes α-Hb, which then leads to oxidation and eventual precipitation of Hb A.
Oxidative stress in AHSP–/– erythrocytes. It is possible that many of the abnormalities observed in AHSP–/– erythroid cells are caused by ROS generated from destabilized α-Hb, a potent oxidant. We investigated this by incubating erythroid cells with 2′,7′-dichlorofluorescin (DCFH), a ROS indicator that is converted to a fluorescent product upon oxidation. As predicted, mutant erythrocytes contained increased ROS both at baseline and after challenge with H2O2, a physiological ROS precursor (Figure 5A). One consequence of oxidative damage to proteins is the introduction of carbonyl groups onto amino acid side chains. This can be examined by derivatization of protein-associated carbonyl moieties with 2,4-dinitrophenylhydrazine (DNPH), followed by Western blot analysis (32). Using this approach, we found that AHSP–/– erythrocytes exhibited distinct oxidative damage, as reflected by elevated levels of carbonyl groups on numerous proteins (Figure 5B).
 Figure 5
Figure 5Oxidative stress in AHSP–/– erythrocytes. (A) Relative ROS levels in erythrocytes at baseline and with added H2O2. ROS were measured by incubation of cells with DCFH, which is converted by ROS to the fluorescent product 2′,7′-dichlorofluorescein (DCF). (B) Protein oxidation in erythrocyte lysates. Twenty micrograms of hemolysate was treated with DNPH for derivatization of carbonyl groups (by products of protein oxidation). Protein-associated DNP was detected by Western blotting. An identical blot probed with anti-actin (bottom) indicates equal protein loading in each lane. (C) Susceptibility to phenylhydrazine-induced hemolytic anemia. Drug was administered on days –1 and 0. Blood was first sampled on day 0, then hematocrit (top panel) and reticulocyte counts (bottom panel) were assessed daily until recovery. No abnormalities were detected in AHSP+/– mice in the experiments described in panels A–C (not shown).
Erythrocytes with intrinsically elevated oxidative stress exhibit baseline damage and depletion of endogenous antioxidant defenses and therefore are expected to be more sensitive to injury from external oxidants. To test this in AHSP–/– mice, we challenged them with phenylhydrazine, an oxidant that causes hemolytic anemia. After treatment with this drug, AHSP–/– mice exhibited a greater fall in hematocrit compared to wild-type littermates (Figure 5C, upper panel). Hence, loss of AHSP renders erythrocytes more susceptible to oxidative damage. Of note, the mutant animals responded appropriately to acute anemia by inducing a rapid reticulocyte response (Figure 5C, lower panel). Hence, the partial block to erythroid development in AHSP–/– mice described in Figure 3, above, is not severe enough to impair physiologic responses to moderate acute anemia.
AHSP inhibits ROS production by α-Hb through direct mechanisms. Our prior biochemical studies demonstrated that AHSP binds and stabilizes α-Hb specifically (16, 17). Free α-Hb produces ROS by several known mechanisms, and the resultant oxidative damage to erythroid cells is believed to be a major determinant of pathophysiology in states of α-Hb excess, most importantly β-thalassemia (33). AHSP could limit damage from α-Hb by directly inhibiting its ability to produce ROS.
Heme-containing proteins, including purified α- or β-Hb subunits, catalyze the production of ROS from H2O2, in a process that can be quantified using tetramethyl-p-phenylenediamine (TMPD), a ROS-sensitive dye that absorbs light at 610 nm upon oxidation (34) (Figure 6A). To assess the effects of AHSP on this process, we preincubated heme proteins with recombinant purified AHSP for 30 minutes prior to the addition of H2O2 and TMPD. AHSP, which binds α-Hb at a stoichiometry of 1:1 (16), inhibited ROS production by α-Hb in a dose-dependent fashion. These effects were specific, as AHSP did not inhibit formation of ROS from β-Hb or myoglobin (Figure 6A and not shown). Hence, one mechanism through which AHSP detoxifies α-Hb is to suppress its pro-oxidant activities through direct protein interaction. These findings provide further evidence that elevated ROS in AHSP–/– mice are caused by failure to stabilize free α-Hb rather than by undefined secondary effects independent of AHSP–α-Hb complex formation.
 Figure 6
Figure 6AHSP prevents α-Hb–mediated production of ROS and oxidation of Hb A in solution. (A) α-Hb or β-Hb was preincubated with AHSP at the indicated molar ratios for 30 minutes at 4°C, then was mixed with H2O2 and TMPD, a ROS indicator dye. The rate of TMPD oxidation, shown on the y axis, was measured by spectrophotometry. Graph shows the average value of three experiments. (B) Effect of AHSP on H2O2-induced heme loss from α-Hb in the experiment in A, as measured by reduced absorbance at 412 nm. The molar ratio of α-Hb to AHSP was 1:1 in the sample on the right. (C) Effect of AHSP or control protein SBTI on α-Hb–mediated oxidation of Hb A. Oxidation of Hb A after 30 minutes at 37°C was measured by spectrophotometry. Where indicated, α-Hb was added to Hb A at a molar ratio of 1:8. AHSP or SBTI was preincubated with α-Hb (1:1 molar ratio) for 30 minutes at 4°C before it was added to Hb A.
Oxidation of the heme iron of α-Hb to the ferric form allows it to participate in further redox reactions that can result in protein destabilization and release of the heme moiety. This is reflected by reduced absorbance in the Soret peak at 412 nm (not shown). Preincubation of α-Hb with AHSP inhibited H2O2-induced loss of heme, indicating that protein destabilization associated with the oxidative process was reduced (Figure 6B). This result is in accordance with AHSP-mediated suppression of ROS production by α-Hb (Figure 6A, above) and with our previous findings that AHSP protects α-Hb from oxidant-induced precipitation (17).
It is possible that relatively low levels of unstable α-Hb in AHSP–/– erythrocytes propagate ROS production with consequent oxidation and precipitation of Hb A and other cellular components. This would explain the presence of β-globin precipitates and unstable Hb A in mutant erythrocytes (Figure 4, above). To test this, we examined the ability of free α-Hb to promote Hb A oxidation in solution (Figure 6C). Conversion of Hb A to the oxidized Fe(III) form is accompanied by characteristic absorbance changes in the visible spectrum that we monitored via deconvolution to known extinction coefficient spectra. After 30 minutes of incubation at 37°C, about 35% of oxygenated Hb A was converted to the met form; this was not affected by addition of AHSP (Figure 6C). The addition of substoichiometric free α-Hb (0.125 molar ratio) roughly doubled the amount of Hb A oxidation. Hence, free α-Hb can promote Hb A oxidation, presumably through production of ROS. These findings are consistent with those of Scott et al., who showed that introducing free α-Hb into normal erythrocytes promotes Hb A oxidation (29, 30). Preincubation with AHSP strongly inhibited the ability of free α-Hb to induce oxidation of Hb A. In contrast, soybean trypsin inhibitor (SBTI), a control protein of a size similar to that of AHSP, had no effect. Therefore, AHSP specifically and directly inhibits the oxidation of Hb A by α-Hb. These findings are consistent with the ability of AHSP to inhibit ROS production by α-Hb and also can account for the presence of unstable Hb A in AHSP–/– erythrocytes (Figures 1 and 4, above).
Loss of AHSP exacerbates β-thalassemia. If α-Hb is stabilized by AHSP in vivo, then loss of the latter could worsen β-thalassemia, a disorder in which erythroid cell damage is mediated by the accumulation of free α-Hb. To test this, we mated AHSP knockout mice with thalassemic ones. We used Hbb+/th-3 (β-globin+/th-3) mice, which contain a targeted deletion of the adult β-globin genes b1 (β major) and b2 (β minor) (35). Heterozygous animals exhibit a β-thalassemia intermedia phenotype, while homozygotes die in utero from severe anemia. We mated β-globin+/th-3 heterozygotes to AHSP+/– mice and then interbred the resulting F1 double-heterozygotes. Genetic analysis of the F1 intercrosses, shown in Figure 7 and Table 2, took into account the fact that AHSP and β-globin are physically linked on the same chromosome (indicated in Figure 7A). Fluorescent in situ hybridization using a bacterial artificial chromosome–derived probe localized AHSP to mouse chromosome 7, band F2-F3 (M.J. Weiss, unpublished data), while the β-globin locus resides on mouse chromosome 7, band E2-E3 (http://www.ncbi.nlm.nih.gov/mapview/maps.cgi?org = mouse&chr = 7&maps = ugMm,model,mgi&query = Hbb-b1[SYM]&cmd = focus&fill = 40&size = 40). Parenthetically, AHSP and β-globin are on separate human chromosomes (16 and 11, respectively).
 Figure 7
Figure 7Intercrosses of β-globin+/th-3AHSP+/– double-heterozygous mice. The th-3 mutant β-globin allele represents a targeted deletion of the b1 and b2 adult globin genes; heterozygous animals exhibit β-thalassemia intermedia, while the homozygous state is lethal in utero. (A) Mating strategy. The β-globin and AHSP genes are physically linked on mouse chromosome 7. Double-heterozygous mice used were the F1 progeny of intercrosses of simple AHSP+/– heterozygotes and β-globin+/th-3 heterozygotes, ensuring that the two mutant alleles were on separate chromosomes (trans configuration). The genotype frequencies of live-born offspring resulting from F1 intercrosses are described in Table 2. +, wild-type allele; –, deleted allele. (B) Hematocrits of β-globin–AHSP compound mutant embryos, with genotypes shown below the x axis. For comparison, embryos are grouped according to the presence or absence of thalassemia (β-globin+/th-3 genotype); thalassemic embryos are subdivided into AHSP-null versus AHSP–wild-type or -heterozygous states. Asterisk denotes either + or – alleles for AHSP. Each symbol represents one embryo analyzed: triangles, β-globin+/+ embryos; circles, β-globin+/th-3 with at least one wild-type AHSP allele; diamonds, β-globin+/th-3AHSP–/–. Color coding is used to specify the AHSP genotype within each group: black, +/+; white, +/–; red, –/–. (C) Blood smears of β-globin+/th-3 embryos with +/+ or –/– AHSP genotypes. Most of the erythrocytes are anucleate definitive (fetal liver derived). Nucleated cells: EP, primitive (yolk sac derived); ED, definitive. Among thalassemic embryos, those lacking AHSP exhibited more prominent eosinophilic erythrocyte inclusions (examples marked by double asterisks) and increased circulating ED cells. Original magnification, ×100.
 Table 2
Table 2Genotype distribution of live-born offspring from intercrosses of β-globin+/th-3AHSP+/–compound mutant mice
Among non–β-thalassemic progeny (Table 2), we observed an overall recombination rate of 0.25 between the β-globin and AHSP genes. Our breeding studies described above established that loss of AHSP does not affect viability when β-globin genes are wild-type. Given the number of meiotic recombination events required to generate each AHSP genotype, the distribution of observed offspring was in good agreement with expected values (Table 2). In marked contrast, the observed AHSP genotype distribution deviated significantly from the expected values in β-globin+/th-3 mice (Table 2). Specifically, the AHSP–/– genotype was underrepresented, indicating that loss of AHSP impairs survival of embryos with thalassemia intermedia (β-globin+/th-3 genotype).
To investigate further the interactions between AHSP and β-thalassemia intermedia, we examined β-globin+/th-3AHSP–/– compound mutant embryos at embryonic day 16.5–17, a stage at which erythropoiesis is predominantly fetal liver derived and, therefore, is dependent upon the expression of adult-type β-globin genes. To favor the production of double-mutant embryos, we crossed β-globin+/th-3 mice that were either AHSP+/– or AHSP–/– with β-globin+/+AHSP+/– mice. All embryos with the β-globin+/th-3 genotype were pale (not shown) and anemic (Figure 7B). However, loss of AHSP reduced the mean hematocrit of thalassemic embryos from 27.5% to 22.6%. The double-mutant embryos showed greater variation in hematocrit, with values reaching as low as 16%. Moreover, the blood smears of thalassemic embryos lacking AHSP showed more inclusion bodies in mature erythrocytes and increased numbers of nucleated fetal liver–derived (definitive) erythrocytes, indicative of erythropoietic stress (Figure 7C). Therefore, it is likely that severe anemia impairs the viability of some β-globin+/th-3AHSP–/– embryos, causing this genotype to be under-represented among live-born pups (Figure 7A and Tables 1 and 2, above).
Analysis of late-stage β-globin+/th-3 embryos suggested that loss of AHSP exacerbates β-globin deficiency in definitive erythrocytes (Figure 7B, above). Consistent with this possibility, the AHSP–/– animals with β-thalassemia intermedia that escaped embryonic death and developed into early adulthood were significantly more anemic than those with AHSP+/+ and AHSP+/– genotypes (Table 3). In addition, AHSP–/– thalassemic mice exhibited smaller erythrocytes (decreased MCV) and greater variations in erythrocyte size (RDW) and Hb content (HDW) (Table 3).
Erythrocytes of AHSP–/– thalassemic mice showed more prominent inclusion bodies than did those of animals with thalassemia alone (Figure 8A), similar to what was observed in the embryos (Figure 7B, above). This suggests that loss of AHSP might exacerbate globin precipitation in the setting of α-chain excess. Therefore, we compared membrane skeleton–associated globin chains in mice of various β-globin and AHSP mutant genotypes (Figure 8, B and C). As expected, predominantly α-globin precipitate was present in β-thalassemic erythrocytes. This is due to the unstable nature of free α-Hb combined with relative β-globin deficiency (5). Importantly, α-globin precipitates in β-thalassemic erythrocytes were significantly increased by deficiency in AHSP (Figure 8B, lanes 2 and 3 compared with lanes 4–6, and Figure 8C), further indicating a role for this protein as a molecular chaperone that neutralizes toxic free α-Hb.
 Figure 8
Figure 8AHSP loss exacerbates α-globin precipitation in adult mice with β-thalassemia intermedia. (A) Blood smears of thalassemic mice (β-globin+/th-3) with AHSP+/+ and AHSP–/– genotypes. Eosinophilic inclusions (marked by asterisks) are more prominent in AHSP–/– erythrocytes. Original magnification, ×100. (B) TAU gel analysis of membrane skeleton–associated globin chain precipitates from mice presented in A. Each lane represents membrane skeletons prepared from the same number of erythrocytes. (C) Relative level of α-globin precipitation in thalassemic erythrocytes of different AHSP genotypes. Membrane skeleton–associated α-globin detected in TAU gels, as illustrated in B, was quantified using the NIH IMAGE software program (http://rsb.info.nih.gov/nih-image/). The average signal obtained from β-thalassemic animals with a wild-type AHSP genotype (black bar) was normalized to a value of 1. n = 4 animals of each genotype; **P < 0.01. Heterozygous loss of AHSP had no significant effect on α-globin precipitation in β-thalassemic mice (not shown).
-
Discussion
Erythrocyte precursors synthesize Hb A tetramer extensively, with minimal damage occurring from cytotoxic free heme and globin subunit precursors. Some of the mechanisms that balance Hb A synthesis and protect against toxicity of its components are recognized. For example, increased efficiency of β-globin translation compensates for higher levels of α-globin RNA transcribed from four active genes (36, 37). Evidence for molecular cross-talk to balance Hb A precursor production is also illustrated by two heme sensor proteins. Heme-regulated kinase inhibits globin translation when heme availability is limited (38). Bach I is a nuclear repressor that inhibits β-globin transcription at low heme concentrations (39). In addition, proteolytic pathways to degrade excess free α-Hb are present in erythroid cells (13–15). It is also to be expected that molecular chaperones exist to transiently stabilize Hb A synthetic intermediates prior to their assembly. α-Hb exists as a monomer, rendering it particularly unstable compared with β-Hb and Hb A, which exist mainly as tetramers under physiologic conditions. For this reason, a chaperone for α-Hb would be particularly advantageous; AHSP potentially serves this function.
AHSP is abundant in late-stage erythroid precursors, in which its expression kinetics parallel that of α-globin (40). AHSP specifically binds free α Hb in a fashion that is not expected to interfere with Hb A assembly or function (16). Moreover, AHSP-bound α-Hb is resistant to denaturation and can still be incorporated into Hb A when β-Hb is available (17). These observations are consistent with a model in which AHSP serves as a docking molecule to temporarily bind α-Hb, stabilize its structure, and render it biochemically inert prior to Hb A assembly. Our results here indicate that when AHSP is absent, α-Hb becomes unstable and generates ROS that damage α-Hb itself, Hb A, and other cellular constituents (Figure 9). AHSP could inhibit ROS production from α-Hb by reducing its inherent ability to participate in redox reactions and/or by inhibiting its denaturation and subsequent heme release (reviewed in refs. 7 and 41). In this regard, our recent data indicate that AHSP induces the conversion of α-Hb to a non-reactive form in which all six coordinate positions of heme-bound iron are liganded (42).
 Figure 9
Figure 9Consequences of AHSP loss in erythroid cells. Without AHSP, α-Hb becomes unstable, with an increased propensity to generate ROS. This leads to further damage and to the precipitation of α-Hb and Hb A, as well as to the oxidation of other cellular constituents. In addition, unstable α-Hb degrades, releasing toxic porphyrins, free iron, and denatured α-globin protein chains (not shown). The end result is premature loss of circulating erythroid cells and erythroid precursors.
The toxicities of α-Hb are most thoroughly studied in the context of β-thalassemia, in which accumulation and precipitation of the free α-Hb subunit reduce the survival of circulating erythrocytes and also cause ineffective erythropoiesis associated with impaired viability and apoptosis of erythroid precursors. Similarly, AHSP–/– mice exhibit shortened erythrocyte half-life with globin precipitates and ineffective erythropoiesis. Unpaired α-Hb is a potent oxidant and β-thalassemic erythrocytes exhibit increased ROS, depleted endogenous antioxidants, and pathological oxidation of cellular lipids and proteins (reviewed in ref. 7). AHSP–/– erythrocytes also contain elevated ROS and signs of oxidative damage. Hence, β-thalassemia and AHSP deficiency share many pathological features. Our current findings indicate that the erythroid defects in AHSP–/– mice stem from unstable α-Hb.
Damage to Hb A by ROS generated from unstable α-Hb probably accounts for the presence of α- and β-globin precipitates in equal proportions in the erythrocyte membrane of AHSP–/– mice (Figure 5, above). In contrast, erythrocytes from mice and humans with β-thalassemia contain mainly α-globin precipitates (24, 43–46), despite high levels of ROS that might be expected to damage other expressed hemoglobins. This is due to excess free α-globin caused by β-globin deficiency (5). In our studies, erythrocyte β-globin precipitates were present in some mice with β-thalassemia intermedia alone, although the relative levels of α-globin precipitates were always greater (Figure 8B and not shown). Concomitant AHSP deficiency exacerbated both α-globin precipitation and, to a lesser extent, β-globin precipitation in thalassemic mice. Therefore, while unstable α-Hb contributes greatly to the pathophysiology of both AHSP deficiency and β-thalassemia, impaired β-globin synthesis in the latter disorder results in a greater proportion of α-globin precipitation.
Our findings that AHSP protects against α-Hb–associated toxicities have potential implications for human disease. For example, defects in AHSP could be responsible for unexplained cases of Heinz body hemolytic anemia in humans. More importantly, variations in AHSP levels could modulate the severity of β-thalassemia in humans. In support of this, an abstract by Galanello et al. reported that reduced AHSP mRNA expression was associated with more severe β-thalassemia among related individuals with the same mutant β-globin alleles, although no mutations in the coding region or proximal promoter of the AHSP gene were detected (47). However, in another recent study, we mapped and sequenced the AHSP gene in 120 Thai patients with HbE–β-thalassemia of varying clinical phenotypes (48). We found no mutations or specific association between AHSP haplotypes and thalassemia severity, indicating that AHSP gene mutations are unlikely to influence thalassemia phenotypes in this population. Whether mutations in the AHSP gene or epigenetic events that alter its expression represent major modifiers of human β-thalassemia still remains an open question. To answer this, it will be important to study the AHSP gene, AHSP mRNA, and AHSP protein in additional β-thalassemic populations and pedigrees. Finally, given that many adverse effects of free α-Hb are relieved by AHSP, it is possible that the pursuit of drugs or peptides that mimic its activities will yield new pharmacological strategies for the treatment of β-thalassemia.
-
Methods
Animals. The generation and genotyping of AHSP-deficient mice was described previously (17). The β-thalassemic mice (th-3 allele) (35) were provided by Oliver Smithies (University of North Carolina, Chapel Hill, North Carolina, USA). Protocols were approved by the Institutional Animal Care and Use Committee of The Children’s Hospital of Philadelphia (Philadelphia, Pennsylvania, USA). All animals were studied between 6 and 20 weeks of age.
Hematological analysis. Blood from adult mice was sampled retro-orbitally, anticoagulated with EDTA, and analyzed on a Bayer ADVIA 120 Hematology System. Hematocrit determination of embryos was performed using 2-μl microcapillary tubes to pierce the carotid vessels and collect blood. Care was taken to prevent contamination of the samples with fluid surrounding the embryo. Three tubes were collected for each embryo. The first tube was filled halfway and discarded. Two additional tubes were filled and the hematocrits determined for each were compared. When values from duplicate samples differed by less than 5%, the average was calculated and used for our analysis (Figure 7B, below). When values differed by more than 5% in samples from the same embryo, we considered there to be a sampling error and data were discarded.
For determination of erythrocyte half-life, NHS-biotin was injected intraperitoneally at a dose of 150 mg/kg on two consecutive days (days –2 and –1 prior to analysis beginning at day 0). Five microliters of blood was drawn from the tail vein, incubated with FITC-streptavidin (BD Biosciences–Pharmingen), and analyzed on a FACSCalibur (BD) (18). For phenylhydrazine treatment, a dose of 63 mg/kg was injected intraperitoneally. Hematocrit and reticulocyte counts were analyzed for 9 days afterward.
Methylcellulose colony assays to quantify erythroid progenitors were performed as described (49). Splenocytes or bone marrow cells were plated in triplicate at a density of 105 per 30-mm dish. Cytokine combinations for specific progenitors were as follows: for CFUe, 2 U/ml Epo; for BFUe, 2 U/ml Epo and 50 ng/ml SCF; for myeloid, 1 ng/ml interleukin 3 (IL-3), 15 U/ml GM-CSF, and 100 U/ml M-CSF. Recombinant human Epo was purchased from Amgen. All other cytokines were mouse reagents and were purchased from R&D Systems.
Proteins. Recombinant AHSP and hemoglobins were purified as described (16).
Analysis of globin precipitates. Heinz body staining was performed as described previously (50). For globin chain analysis in membrane skeletons (51), 40 μl of freshly drawn blood was lysed and washed extensively in 0.05% PBS. Membrane lipids were removed by extraction with 56 mM sodium borate (pH 8.0). Precipitated globins were dissolved in 8 M urea, 10% acetic acid, 10% β-mercaptoethanol, and 0.04% pyronin, were fractionated on TAU gels, and stained with Coomassie brilliant blue. The fraction of sample loaded on the TAU gel was adjusted relative to the original hematocrit so that equal numbers of erythrocytes were represented in each lane.
Flow cytometry. Analysis to quantify erythroid maturation stages in spleen and bone marrow was performed as described previously (19). Single-cell suspensions were stained with PE-Ter119 and FITC-CD71 (BD Biosciences — Pharmingen). For exclusion of dead cells, 7-aminoactinomycin D (Sigma) was used. Studies were performed on a FACSCalibur System (BD). For detection of intracellular ROS, 107 peripheral blood cells were pre-loaded with DCFH (Sigma) and then were incubated with H2O2 as indicated in Figure 5A, and intracellular fluorescence intensity was detected by flow cytometry (52).
Detection of carbonyl-modified intracellular proteins. Protein was prepared from mouse erythrocytes and 20 μg was analyzed with the Oxyblot Kit (Intergen) following the manufacturer’s protocol.
Tissue collection and histopathology. Tissues for immunohistochemistry and histopathology were fixed in formalin, embedded in paraffin, and sectioned using standard procedures. Immunohistochemistry was performed using Ventanna systems. The TUNEL assay was performed in accordance with the manufacturer’s recommendations (Roche). A monoclonal antibody (LY-76; BD Biosciences–Pharmingen) against the Ter119 antigen was used to identify late-stage erythroid cells. Double labeling was accomplished with a combination of both techniques.
ROS production by α-Hb. A solution of 40 μM oxygenated α-Hb with or without 40 μM AHSP was pre-incubated on ice for 30 minutes. TMPD (Sigma) (34) was then added to a final concentration of 400 μM and its oxidation was measured by continuous light absorbance monitoring at 610 nm. The rate of oxidation was calculated based on the slope of the initial 10 minutes of the reaction. Heme loss was measured by the decrease of light absorbance in the Soret range (412 nm).
Hb A oxidation induced by its subunits. Oxygenated α-Hb (5 μM) was incubated on ice for 30 minutes with 5 μM AHSP [or SBTI (Sigma) as negative control] to form the α-Hb–AHSP complex. This complex was added to a mixture of 40 μM oxygenated Hb A and 1 mM reduced glutathione (28). The reaction was incubated at 37°C for 30 minutes and the fraction of Fe(III) Hb was calculated by linear regression to extinction coefficient spectra over the wavelength range of 500–700 nm (53).
Statistical methods. The unpaired 2-sample Student’s t test was used for statistical analysis in Figures 1–8 and in Tables 1 and 3. A P value of less than 0.05 was considered to be significant. For genetic linkage analysis (Figure 7A and Table 2) we used the chi-squared test.
-
Acknowledgments
This work was funded by the NIH National Institute of Diabetes and Digestive and Kidney Diseases (to M.J. Weiss) and the Cooley’s Anemia Foundation (to S. Zhou and M.J. Weiss). Y. Kong is the recipient of an American Heart Association Predoctoral Fellowship. We thank Deanna Ramirez-Bergeron for assistance in embryo analysis and Richard Spielman and David Nathan for helpful discussions and for reviewing the manuscript.
-
Footnotes
Anthony J. Kihm’s present address is: Johnson & Johnson, Somerville, New Jersey, USA.
Nonstandard abbreviations used: AHSP, α-Hb–stabilizing protein; BFUe, burst-forming unit erythroid; CFUe, CFU erythroid; DCFH, 2′,7′-dichlorofluorescin; DNPH, 2,4-dinitrophenylhydrazine; Epo, erythropoietin; Hb, hemoglobin; HDW, Hb distribution width; MCH, mean corpuscular Hb; MCV, mean corpuscular volume; NHS, N-hydroxysuccinimide; RDW, rbc distribution width; SBTI, soybean trypsin inhibitor; SCF, stem cell factor; TAU, Triton–acetic acid–urea; TMPD, tetramethyl-p-phenylenediamine.
Conflict of interest: The authors have declared that no conflict of interest exists.
-
References
- Rachmilewitz, E., and Schrier, S.L. 2001. Pathophysiology of β thalassemia. In Disorders of hemoglobin: genetics, pathophysiology, and clinical management. M.H. Steinberg, B.G. Forget, D.R. Higgs, and R.L. Nagel, editors. Cambridge University Press. Cambridge, United Kingdom. 233–251.
- Nathan, DG, Gunn, RB. Thalassemia: the consequences of unbalanced hemoglobin synthesis. Am. J. Med. 1966. 41:815-830.
- Baglioni, C. Chromosomal and cytoplasmic regulation of haemoglobin synthesis. Bibl. Haematol. 1966. 29:1056-1063.
- Bank, A. Hemoglobin synthesis in beta-thalassemia: the properties of the free alpha-chains. J. Clin. Invest. 1968. 47:860-866.
- Bank, A, Marks, PA. Excess alpha chain synthesis relative to beta chain synthesis in thalassaemia major and minor. Nature. 1966. 212:1198-1200. View this article via: CrossRef Google Scholar
- Brunori, M, Falcino, G, Fioreti, E. Formation of superoxide in the autoxidation of the isolated alpha and beta chains of human hemoglobin and its involvement in hemichrome precipitation. Eur. J. Biochem. 1975. 53:99-104. View this article via: CrossRef Google Scholar
- Shinar, E, Rachmilewitz, EA. Oxidative denaturation of red blood cells in thalassemia. Semin. Hematol. 1990. 27:70-82. View this article via: PubMed Google Scholar
- Wickner, S, Maurizi, MR, Gottesman, S. Posttranslational quality control: folding, refolding, and degrading proteins. Science. 1999. 286:1888-1893.
- Shaeffer, JR. Evidence for soluble alpha-chains as intermediates in hemoglobin synthesis in the rabbit reticulocyte. Biochem. Biophys. Res. Commun. 1967. 28:647-652.
- Tavill, AS, Grayzel, AI, Vanderhoff, GA, London, IM. The control of hemoglobin synthesis. Trans. Assoc. Am. Physicians. 1967. 80:305-313. View this article via: PubMed Google Scholar
- Weatherall, DJ. Phenotype-genotype relationships in monogenic disease: lessons from the thalassaemias. Nat. Rev. Genet. 2001. 2:245-255.
- Bank, A, O’Donnell, JV. Intracellular loss of free alpha chains in beta thalassemia. Nature. 1969. 222:295-296.
- Shaeffer, JR. Turnover of excess hemoglobin alpha chains in beta-thalassemic cells is ATP-dependent. J. Biol. Chem. 1983. 258:13172-13177. View this article via: PubMed Google Scholar
- Shaeffer, JR, Cohen, RE. Enhancement by ubiquitin aldehyde of proteolysis of hemoglobin alpha-subunits in beta-thalassemic hemolysates. Ann. N. Y. Acad. Sci. 1998. 850:394-397.
- Shaeffer, JR, Kania, MA. Degradation of monoubiquitinated alpha-globin by 26S proteasomes. Biochemistry. 1995. 34:4015-4021.
- Gell, D, Kong, Y, Eaton, SA, Weiss, MJ, Mackay, JP. Biophysical characterization of the alpha-globin binding protein alpha-hemoglobin stabilizing protein. J. Biol. Chem. 2002. 277:40602-40609.
- Kihm, AJ, et al. An abundant erythroid protein that stabilizes free alpha hemoglobin. Nature. 2002. 417:758-763.
- Hoffmann-Fezer, G, et al. Biotin labeling as an alternative nonradioactive approach to determination of red cell survival. Ann. Hematol. 1993. 67:81-87.
- Socolovsky, M, et al. Ineffective erythropoiesis in Stat5a(–/–)5b(–/–) mice due to decreased survival of early erythroblasts. Blood. 2001. 98:3261-3273.
- Joshi, W, Leb, L, Piotrowski, J, Fortier, N, Snyder, LM. Increased sensitivity of isolated alpha subunits of normal human hemoglobin to oxidative damage and crosslinkage with spectrin. J. Lab. Clin. Med. 1983. 102:46-52. View this article via: PubMed Google Scholar
- Weatherall, DJ, Clegg, JB, Na-Nakorn, S, Wasi, P. The pattern of disordered haemoglobin synthesis in homozygous and heterozygous beta-thalassaemia. Br. J. Haematol. 1969. 16:251-267.
- Shinar, E, Rachmilewitz, EA, Lux, SE. Differing erythrocyte membrane skeletal protein defects in alpha and beta thalassemia. J. Clin. Invest. 1989. 83:404-410.
- Shinar, E, Shalev, O, Rachmilewitz, EA, Schrier, SL. Erythrocyte membrane skeleton abnormalities in severe beta-thalassemia. Blood. 1987. 70:158-164. View this article via: PubMed Google Scholar
- Rouyer-Fessard, P, et al. A study of membrane protein defects and alpha hemoglobin chains of red blood cells in human beta thalassemia. J. Biol. Chem. 1989. 264:19092-19098. View this article via: PubMed Google Scholar
- Schrier, SL, Rachmilewitz, E, Mohandas, N. Cellular and membrane properties of alpha and beta thalassemic erythrocytes are different: implication for differences in clinical manifestations. Blood. 1989. 74:2194-2202. View this article via: PubMed Google Scholar
- Yuan, J, Kannan, R, Shinar, E, Rachmilewitz, EA, Low, PS. Isolation, characterization, and immunoprecipitation studies of immune complexes from membranes of beta-thalassemic erythrocytes. Blood. 1992. 79:3007-3013. View this article via: PubMed Google Scholar
- Advani, R, et al. Characterization and comparison of the red blood cell membrane damage in severe human alpha- and beta-thalassemia. Blood. 1992. 79:1058-1063. View this article via: PubMed Google Scholar
- Scott, MD, Eaton, JW. Thalassaemic erythrocytes: cellular suicide arising from iron and glutathione-dependent oxidation reactions? Br. J. Haematol. 1995. 91:811-819.
- Scott, MD, Rouyer-Fessard, P, Lubin, BH, Beuzard, Y. Entrapment of purified alpha-hemoglobin chains in normal erythrocytes. A model for beta thalassemia. J. Biol. Chem. 1990. 265:17953-17959. View this article via: PubMed Google Scholar
- Scott, MD, et al. Effect of excess alpha-hemoglobin chains on cellular and membrane oxidation in model beta-thalassemic erythrocytes. J. Clin. Invest. 1993. 91:1706-1712.
- Kim, H.C., and Schwartz, E. 1995. Unstable hemoglobins. In Williams hematology. E. Buetler, M.A. Lichtman, B.S. Coller, and T.J. Kipps, editors. McGraw-Hill. New York, New York, USA. L33–L34.
- Nakamura, A, Goto, S. Analysis of protein carbonyls with 2,4-dinitrophenyl hydrazine and its antibodies by immunoblot in two-dimensional gel electrophoresis. J. Biochem. 1996. 119:768-774. View this article via: PubMed Google Scholar
- Schrier, SL. Pathophysiology of thalassemia. Curr. Opin. Hematol. 2002. 9:123-126.
- Van der Ouderaa, FJ, Buytenhek, M, Nugteren, DH, Van Dorp, DA. Purification and characterisation of prostaglandin endoperoxide synthetase from sheep vesicular glands. Biochim. Biophys. Acta. 1977. 487:315-331. View this article via: PubMed Google Scholar
- Yang, B, et al. A mouse model for beta 0-thalassemia. Proc. Natl. Acad. Sci. U. S. A. 1995. 92:11608-11612.
- Lodish, HF, Jacobsen, M. Regulation of hemoglobin synthesis. Equal rates of translation and termination of α- and β-globin chains. J. Biol. Chem. 1972. 247:3622-3629. View this article via: PubMed Google Scholar
- Nathan, DG, Lodish, HF, Kan, YW, Housman, D. Beta thalassemia and translation of globin messenger RNA. Proc. Natl. Acad. Sci. U. S. A. 1971. 68:2514-2518.
- Han, AP, et al. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 2001. 20:6909-6918.
- Tahara, T, et al. Heme positively regulates the expression of b-globin at the locus control region via the transcriptional factor Bach1 in erythroid cells. J. Biol. Chem. 2004. 279:5480-5487.
- dos Santos, CO, Duarte, ASS, Saad, STO, Costa, FF. Expression of alpha hemoglobin stabilizing protein gene during human erythropoiesis. Exp. Hematol. 2004. 32:157-162.
- Bunn, H.F., and Forget, B.G. 1986. Unstable hemoglobin variants-congenital Heinz body hemolytic anemia. In Hemoglobin: molecular, genetic, and clinical aspects. H.F. Bunn, and B.G. Forget, editors. W.B. Saunders. Philadelphia, Pennsylvania, USA. 565–594.
- Feng, L., et al. 2004. Molecular mechanisms of AHSP-mediated stabilization of hemoglobin. Cell. In press.
- Fessas, P. Inclusions of hemoglobin erythroblasts and erythrocytes of thalassemia. Blood. 1963. 21:21-32. View this article via: PubMed Google Scholar
- Fessas, P, Loukopoulos, D, Kaltsoya, A. Peptide analysis of the inclusions of erythroid cells in beta-thalassemia. Biochim. Biophys. Acta. 1966. 124:430-432. View this article via: PubMed Google Scholar
- Beauchemin, H, Blouin, MJ, Trudel, M. Differential regulatory and compensatory responses in hematopoiesis/erythropoiesis in α and β globin hemizygous mice. J. Biol. Chem. 2004. 279:19471-19480.
- Rouyer-Fessard, P, Leroy-Viard, K, Domenget, C, Mrad, A, Beuzard, Y. Mouse beta thalassemia, a model for the membrane defects of erythrocytes in the human disease. J. Biol. Chem. 1990. 265:20247-20251. View this article via: PubMed Google Scholar
- Galanello, R, Perseu, L, Giagu, N, Sole, G. AHSP expression in beta-thalassemia carriers with thalassemia intermedia phenotype [abstract]. Blood. 2003. 102:1881.
- Viprakasit, V, et al. Evaluation of alpha hemoglobin stabilizing protein (AHSP) as a genetic modifier in patients with {beta} thalassemia. Blood. 2004. 103:3296-3299.
- Keller, G, Kennedy, M, Papayannopoulou, T, Wiles, MV. Hematopoietic differentiation during embryonic stem cell differentiation in culture. Mol. Cell. Biol. 1993. 13:472-486.
- Stamatoyannopoulos, G, Woodson, R, Papayannopoulou, T, Heywood, D, Kurachi, S. Inclusion-body beta-thalassemia trait. A form of beta thalassemia producing clinical manifestations in simple heterozygotes. N. Engl. J. Med. 1974. 290:939-943. View this article via: PubMed Google Scholar
- Sorensen, S, Rubin, E, Polster, H, Mohandas, N, Schrier, S. The role of membrane skeletal-associated alpha-globin in the pathophysiology of beta-thalassemia. Blood. 1990. 75:1333-1336. View this article via: PubMed Google Scholar
- Zhu, H, Bannenberg, GL, Moldeus, P, Shertzer, HG. Oxidation pathways for the intracellular probe 2′,7′-dichlorofluorescein. Arch. Toxicol. 1994. 68:582-587.
- Gow, AJ, Luchsinger, BP, Pawloski, JR, Singel, DJ, Stamler, JS. The oxyhemoglobin reaction of nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 1999. 96:9027-9032.
-
Version history
- Version 1 (November 15, 2004): No description



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)












