Advertisement
Spotlight Free access | 10.1172/JCI16421
Isoprenoids as mediators of the biological effects of statins
James K. Liao
Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA
Address correspondence to: James K. Liao, Vascular Medicine Research, Brigham and Women’s Hospital, 65 Landsdowne Street, Room 275, Cambridge, Massachusetts 02139, USA. Phone: (617) 768-8424; Fax: (617) 768-8425; E-mail: jliao@rics.bwh.harvard.edu.
Find articles by Liao, J. in: PubMed | Google Scholar
Published August 1, 2002 - More info
J Clin Invest. 2002;110(3):285–288. https://doi.org/10.1172/JCI16421.
© 2002 The American Society for Clinical Investigation
The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, or statins, are potent inhibitors of cholesterol biosynthesis. Several large clinical trials have demonstrated the benefits of cholesterol lowering with these agents in the primary and secondary prevention of coronary heart disease. The overall clinical benefits observed with statin therapy, however, appear to be greater than what might be expected from changes in lipid profile alone, suggesting that the beneficial effects of statins may extend beyond their effects on serum cholesterol levels.
Recent experimental and clinical evidence indicates that some of the cholesterol-independent, or so-called pleiotropic, effects of statins involve improving or restoring endothelial function, enhancing the stability of atherosclerotic plaques, decreasing oxidative stress and inflammation, and inhibiting the thrombogenic response in the vascular wall. Many of these cholesterol-independent effects reflect statins’ ability to block the synthesis of important isoprenoid intermediates, which serve as lipid attachments for a variety of intracellular signaling molecules. In particular, the inhibition of small GTP-binding proteins Rho, Ras, and Rac, whose proper membrane localization and function are dependent upon isoprenylation, may play an important role in mediating the biological effects of statins.
-
Pharmacological properties of statins
Statins bind to HMG-CoA reductase at nanomolar concentrations, leading to competitive displacement of the natural substrate, HMG-CoA, which binds at micromolar concentrations (1). In addition, inhibition of cholesterol biosynthesis is accompanied by an increase in hepatic LDL receptor, which promotes uptake and clearance of cholesterol from the bloodstream. While all statins inhibit hepatic HMG-CoA reductase to varying degrees, important structural differences exist among the statins that distinguish their lipophilicity, half-life, and potency (2). For example, one of the more potent newer statins, rosuvastatin, is relatively hydrophilic and has a greater number of bonding interactions with the catalytic site of HMG-CoA reductase compared with mevastatin, fluvastatin, simvastatin, cerivastatin, and atorvastatin (1, 3).
The lipophilic statins would be expected to penetrate cell membranes more effectively than the more hydrophilic statins, causing more side effects but, at the same time, eliciting more pleiotropic effects. However, the observation that hydrophilic statins have pleiotropic effects similar to those of lipophilic statins calls into question whether there are really any cholesterol-independent effects of statins. Indeed, recent evidence suggests that some of the cholesterol-independent effects of these agents may be mediated by inhibition of hepatic HMG-CoA reductase, leading to subsequent reduction in circulating isoprenoid levels (4). This hypothesis may help explain why hydrophilic statins such as pravastatin and rosuvastatin are still able to exert cholesterol-independent benefits on the vascular wall without directly entering vascular wall cells. In this respect, the word “pleiotropic” probably does not reflect the hepatic versus nonhepatic effects of these agents.
-
Clinical trials with statins
Because serum cholesterol level is strongly associated with coronary heart disease (5), it has been generally assumed that cholesterol reduction by statins is the predominant, if not the only, mechanism underlying their beneficial effects in cardiovascular diseases. However, subgroup analysis of large clinical trials such as the 4S, WOSCOP, CARE, and HPS suggests that the clinical benefits of statins are not associated with base-line cholesterol levels or the degree of cholesterol reduction (6–9). Furthermore, in angiographic trials, clinical improvements with statins far exceed changes in the size of atherosclerotic lesions (10). It is quite likely that cholesterol lowering in these long-term trials stabilized atherosclerotic plaques and made them less prone to rupture. However, in the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) trial, statins reduced recurrent ischemic events within 16 weeks following acute coronary ischemia (11). Although the serum LDL-cholesterol was decreased by 40%, this time frame was probably far too rapid for appreciable changes in lesion size and plaque stability to occur as a consequence of cholesterol reduction.
An intriguing but perplexing result of large clinical trials with statins is the reduction in ischemic stroke (12). Although myocardial infarction is closely associated with serum cholesterol levels, neither the Framingham Heart Study nor the Multiple Risk Factor Intervention Trial (MRFIT) demonstrated significant correlation between ischemic stroke and serum cholesterol levels (13, 14). Thus, the findings of these large statin trials raise the interesting question of how statins could reduce ischemic stroke when ischemic stroke and cholesterol are unrelated. It appears likely that some of the beneficial effects of statins in ischemic stroke are attributable to the pleiotropic effects of statins on endothelial function and fibrinolytic pathways.
-
Statins and isoprenylated proteins
By inhibiting L-mevalonic acid synthesis, statins also prevent the synthesis of other important isoprenoid intermediates of the cholesterol biosynthetic pathway, such as farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP) (15) (Figure 1). These intermediates serve as important lipid attachments for the posttranslational modification of a variety of cell-signaling proteins. Protein isoprenylation permits the covalent attachment, subcellular localization, and intracellular trafficking of membrane-associated proteins (16). Members of the Ras and Rho GTPase family are major substrates for posttranslational modification by isoprenylation and may be important targets for inhibition by statins. Indeed, statins induce changes in the actin cytoskeleton and assembly of focal adhesion complexes by inhibiting RhoA and Rac1 isoprenylation (Figure 2).
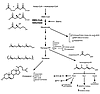 Figure 1
Figure 1Biological actions of isoprenoids and cholesterol. This diagram of the cholesterol biosynthesis pathway shows the effects of inhibition of HMG-CoA reductase by statins. Decrease in isoprenylation of signaling molecules such as Ras, Rho, and Rac leads to modulation of various signaling pathways. BMP-2, bone morphogenetic protein-2; eNOS, endothelial nitric oxide synthase; t-PA, tissue-type plasminogen activator; ET-1, endothelin-1; PAI-1, plasminogen activator inhibitor-1.
 Figure 2
Figure 2Actin cytoskeletal effects of statins. Phalloidin staining of human endothelial cells shows the effects of the statin simvastatin (10 μM) on actin stress fibers and focal adhesion complexes (green) with and without L-mevalonate (L-Mev, 200 μM).
Besides altering the actin cytoskeleton, inhibition of RhoA by statins increases endothelial nitric oxide synthase (eNOS) expression and decreases severity of cerebral ischemia in a mouse model of ischemic stroke (17, 18). Similarly, statins also increase the expression of tissue-type plasminogen activator (19) and inhibit the expression of plasminogen activator inhibitor-1 (19) and endothelin-1 by mechanisms involving inhibition of geranylgeranylation (20). Because Ras and Rho also regulate the cell cycle, they are, in addition, likely targets for the direct antiproliferative effects of statins. Indeed, statins inhibit vascular smooth muscle cell proliferation in transplant-associated arteriosclerosis (21) and may have clinical benefits in inhibiting certain breast cancers (22). Finally, inhibition of Rac1 geranylgeranylation and Rac1-mediated NAD(P)H oxidase activity by statins attenuates angiotensin II–induced reactive oxygen species production in vascular smooth muscle cells and cardiac myocytes (23, 24) (Figure 3). These cholesterol-independent antioxidant effects of statins lead to the inhibition of hypertrophic responses in these tissues.
 Figure 3
Figure 3Antioxidant effects of statins. Intracellular oxidation (red) as determined by 2′,7′-dichlorofluoroscein staining of rat cardiomyocytes treated with angiotensin II (Ang II, 10 nM), with and without simvastatin (statin, 10 μM), L-mevalonate (L-Mev, 200 μM), GGPP (100 μM), or FPP (100 μM).
-
Statins and cardiovascular diseases
Plaque rupture is a major cause of acute coronary syndromes (25). Lipid lowering by statins contributes to plaque stability by reducing plaque size or by modifying the physiochemical properties of the lipid core. However, since the changes in plaque size associated with lipid lowering tend to occur over extended time and to be quite minimal as assessed by angiography, it appears that the clinical benefits from statins must have another explanation. Most likely, these benefits arise from a combined reduction in lipids and macrophage accumulation in atherosclerotic lesions and inhibition of matrix metalloproteinases and tissue factor production by activated macrophages (26, 27).
Recently, statins have been found to increase the number of circulating endothelial progenitor cells (EPCs), which may give rise to neovascularization in ischemic tissues (28). Indeed, statin therapy induces angiogenesis by promoting the proliferation, migration, and survival of circulating EPCs via the phosphatidylinositol (PI) 3-kinase/Akt pathway (29). In patients with angiographically documented, stable coronary artery disease, statins augment the number of circulating EPCs and enhanced functional activity (30). These findings agree with earlier data showing that statin therapy rapidly activates PI 3-kinase/Akt and eNOS, inhibits apoptosis, and accelerates vascular structure formation (31). Interestingly, these angiogenic effects occur rapidly at very low concentrations of statins and are cholesterol-independent.
-
Are clinical benefits of statin therapy due entirely to cholesterol lowering?
Many clinicians, especially lipidologists, find it difficult to embrace the concept of statin pleiotropy for a number of reasons. First, patients receiving statin therapy invariably will have reduced lipid levels, and it is often difficult to separate the lipid-lowering from the non–lipid-lowering effects of statins in clinical trials. Second, many effects of statins, such as improvement in endothelial function, decreased inflammation, increased plaque stability, and reduced thrombogenic response, could all be accounted for, to some extent, by lipid lowering. Third, the concentrations used to demonstrate the biological effects of statins in cell culture and animal experiments, especially with regard to inhibition of Rho geranylgeranylation (but not PI 3-kinase/Akt activation), appear to be much higher than those prescribed clinically. Finally, both hydrophilic and lipophilic statins, which inhibit hepatic HMG-CoA reductase, appear to exert similar cholesterol-independent effects, despite the relative impermeability of hydrophilic statins in vascular tissues. Thus, it appears that statins are very potent cholesterol-lowering agents and that reduction in cholesterol levels by statins contributes to many of their clinical benefits.
The evidence for cholesterol-independent effects of statins in humans, however, stems mostly from the rapidity of statin action in clinical trials (i.e., sometimes within days) and from evidence for clinical benefits that are not related to base-line cholesterol levels or the degree of cholesterol reduction. Furthermore, statins appear to exert clinical benefits beyond cardiovascular disease, including a reduction in the risk of dementia (32), Alzheimer disease (33), ischemic stroke (12), osteoporosis (34), and possibly breast cancer (22). Indeed, there is a growing body of biological, epidemiological, and limited but nonrandomized clinical evidence indicating that lowering serum cholesterol by statins may retard the pathogenesis of Alzheimer disease (35). Because neurons receive only small amounts of exogenous cholesterol, statins that reduce endogenous isoprenoid and cholesterol synthesis may inhibit the formation of Aβ-amyloid peptide by removing amyloid precursor protein from cholesterol- and sphingolipid-enriched membrane microdomains (36). However, in a recent prospective study, lipid and lipoprotein levels were not associated with the development of Alzheimer disease (37). These interesting observations suggest that the cellular or non–cholesterol-lowering effects of statins may be more important in influencing the progression of Alzheimer disease.
For osteoporosis, ischemic stroke, and other conditions for which statins appear to be beneficial, there is no clear association between cholesterol levels and risk of disease. Is it possible, then, that in normocholesterolemic individuals or in patients with ischemic stroke, plasma cholesterol, like L-mevalonate, is merely a marker of statins’ inhibitory effect on HMG-CoA reductase, rather than the cause of the disease? Perhaps in patient populations where cholesterol is not an overt risk factor, other factors such as inflammation, which is also reduced by statin therapy, may be a more appropriate marker of statin efficacy than serum cholesterol levels (38). These uncertainties beg for further randomized clinical trials that would allow the cholesterol-dependent and -independent effects of statins to be evaluated separately. Only then will one be able to determine conclusively whether real clinical benefits of statin therapy beyond lipid lowering exist.
-
References
- Istvan, ES, Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001. 292:1160-1164.
- Illingworth, DR, Tobert, JA. HMG-CoA reductase inhibitors. Adv Protein Chem 2001. 56:77-114.
- McTaggart, F, et al. Preclinical and clinical pharmacology of Rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol 2001. 87:28B-32B. View this article via: PubMed Google Scholar
- Corsini, A, et al. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther 1999. 84:413-428.
- Klag, MJ, et al. Serum cholesterol in young men and subsequent cardiovascular disease. N Engl J Med 1993. 328:313-318.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994. 344:1383-1389. View this article via: PubMed Google Scholar
- Shepherd, J, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995. 333:1301-1307.
- Sacks, FM, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996. 335:1001-1009.
- Collins, R, Peto, R, Armitage, J. The MRC/BHF Heart Protection Study: preliminary results. Int J Clin Pract 2002. 56:53-56. View this article via: PubMed Google Scholar
- Brown, BG, Zhao, XQ, Sacco, DE, Albers, JJ. Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation 1993. 87:1781-1791. View this article via: PubMed Google Scholar
- Schwartz, GG, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL study: a randomized controlled trial. JAMA 2001. 285:1711-1718.
- Crouse, JR, Byington, RP, Furberg, CD. HMG-CoA reductase inhibitor therapy and stroke risk reduction: an analysis of clinical trials data. Atherosclerosis 1998. 138:11-24.
- Kannel, WB, Castelli, WP, Gordon, T, McNamara, PM. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med 1971. 74:1-12. View this article via: PubMed Google Scholar
- Multiple risk factor intervention trial: risk factor changes and mortality results. Multiple Risk Factor Intervention Trial Research Group. JAMA 1982. 248:1465-1477.
- Goldstein, JL, Brown, MS. Regulation of the mevalonate pathway. Nature 1990. 343:425-430.
- Van Aelst, L, D’Souza-Schorey, C. Rho GTPases and signaling networks. Genes Dev 1997. 11:2295-2322.
- Endres, M, et al. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA 1998. 95:8880-8885.
- Laufs, U, et al. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J Clin Invest 2000. 106:15-24.
- Essig, M, et al. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors increase fibrinolytic activity in rat aortic endothelial cells. Role of geranylgeranylation and Rho proteins. Circ Res 1998. 83:683-690. View this article via: PubMed Google Scholar
- Hernandez-Perera, O, Perez-Sala, D, Soria, E, Lamas, S. Involvement of rho GTPases in the transcriptional inhibition of preproendothelin-1 gene expression by simvastatin in vascular endothelial cells. Circ Res 2000. 87:616-622. View this article via: PubMed Google Scholar
- Kobashigawa, JA, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 1995. 333:621-627.
- Denoyelle, C, et al. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis 2001. 22:1139-1148.
- Wassmann, S, et al. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol 2001. 59:646-654. View this article via: PubMed Google Scholar
- Takemoto, M, et al. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest 2001. 108:1429-1437. doi:10.1172/JCI200113350.
- Libby, P. Molecular bases of the acute coronary syndromes. Circulation 1995. 91:2844-2850. View this article via: PubMed Google Scholar
- Aikawa, M, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation 2001. 103:276-283. View this article via: PubMed Google Scholar
- Crisby, M, et al. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 2001. 103:926-933. View this article via: PubMed Google Scholar
- Llevadot, J, et al. HMG-CoA reductase inhibitor mobilizes bone marrow–derived endothelial progenitor cells. J Clin Invest 2001. 108:399-405. doi:1172/JCI200113131.
- Dimmeler, S, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest 2001. 108:391-397. doi:1172/JCI200113152.
- Vasa, M, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 2001. 103:2885-2890.
- Kureishi, Y, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normo-cholesterolemic animals. Nat Med 2000. 6:1004-1010.
- Jick, H, Zornberg, GL, Jick, SS, Seshadri, S, Drachman, DA. Statins and the risk of dementia. Lancet 2000. 356:1627-1631.
- Wolozin, B, Kellman, W, Ruosseau, P, Celesia, GG, Siegel, G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol 2000. 57:1439-1443.
- Chan, KA, et al. Inhibitors of hydroxymethylglutaryl-coenzyme A reductase and risk of fracture among older women. Lancet 2000. 355:2185-2188.
- Scott, H.D., and Laake, K. 2001. Statins for the prevention of Alzheimer’s disease. Cochrane Database Syst. Rev. CD003160.
- Simons, M, Keller, P, Dichgans, J, Schulz, JB. Cholesterol and Alzheimer’s disease: is there a link? Neurology 2001. 57:1089-1093. View this article via: PubMed Google Scholar
- Moroney, JT, et al. Low-density lipoprotein cholesterol and the risk of dementia with stroke. JAMA 1999. 282:254-260.
- Ridker, PM, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001. 344:1959-1965.
-
Version history
- Version 1 (August 1, 2002): No description



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)



