Abstract
Serine-rich splicing factor 3 (SRSF3) plays a critical role in liver function and its loss promotes chronic liver damage and regeneration. As a consequence, genetic deletion of SRSF3 in hepatocytes caused progressive liver disease and ultimately led to hepatocellular carcinoma. Here we show that SRSF3 is decreased in human liver samples with nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), or cirrhosis that was associated with alterations in RNA splicing of known SRSF3 target genes. Hepatic SRSF3 expression was similarly decreased and RNA splicing dysregulated in mouse models of NAFLD and NASH. We showed that palmitic acid–induced oxidative stress caused conjugation of the ubiquitin-like NEDD8 protein to SRSF3 and proteasome-mediated degradation. SRSF3 was selectively neddylated at lysine 11 and mutation of this residue (SRSF3-K11R) was sufficient to prevent both SRSF3 degradation and alterations in RNA splicing. Lastly, prevention of SRSF3 degradation in vivo partially protected mice from hepatic steatosis, fibrosis, and inflammation. These results highlight a neddylation-dependent mechanism regulating gene expression in the liver that is disrupted in early metabolic liver disease and may contribute to the progression to NASH, cirrhosis, and ultimately hepatocellular carcinoma.
Authors
Deepak Kumar, Manasi Das, Consuelo Sauceda, Lesley G. Ellies, Karina Kuo, Purva Parwal, Mehak Kaur, Lily Jih, Gautam K. Bandyopadhyay, Douglas Burton, Rohit Loomba, Olivia Osborn, Nicholas J.G. Webster
Figure 5
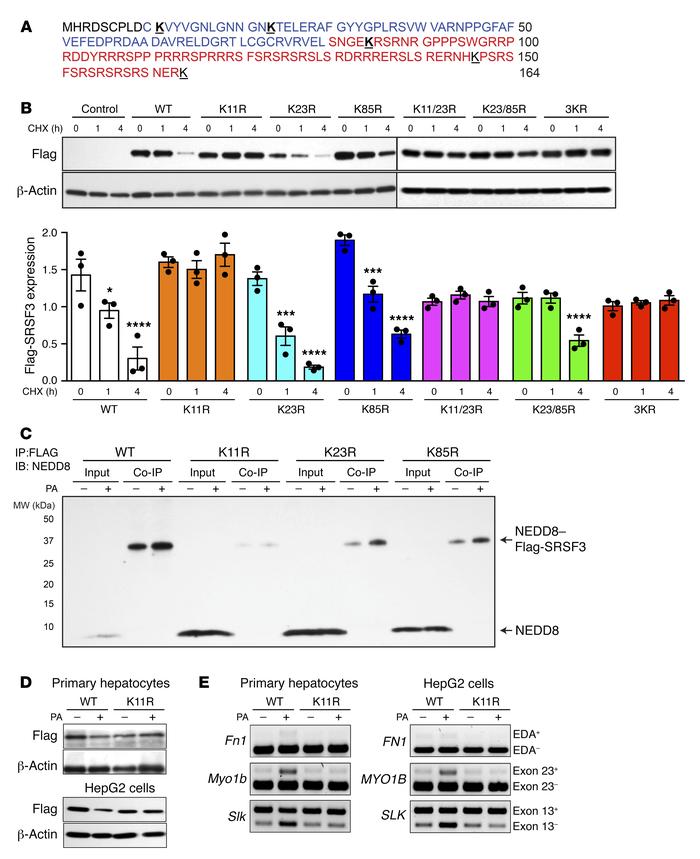
Mutation of lysine 11 prevented SRSF3 protein degradation.



Copyright © 2026 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)