Citation Information: J Clin Invest. 2019;129(2):802-819. https://doi.org/10.1172/JCI122035.
Abstract
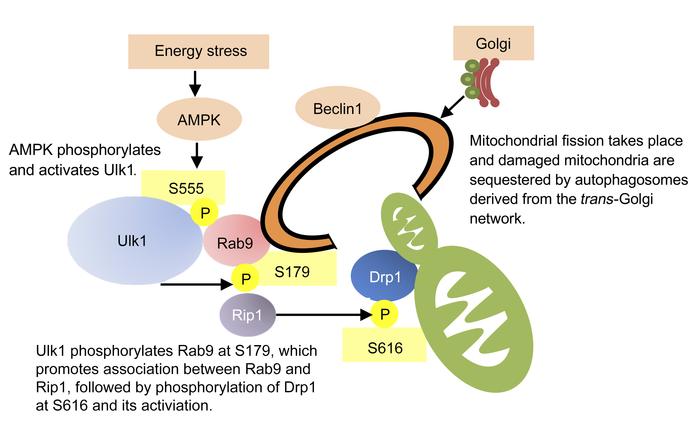
Energy stress, such as ischemia, induces mitochondrial damage and death in the heart. Degradation of damaged mitochondria by mitophagy is essential for the maintenance of healthy mitochondria and survival. Here, we show that mitophagy during myocardial ischemia was mediated predominantly through autophagy characterized by Rab9-associated autophagosomes, rather than the well-characterized form of autophagy that is dependent on the autophagy-related 7 (Atg) conjugation system and LC3. This form of mitophagy played an essential role in protecting the heart against ischemia and was mediated by a protein complex consisting of unc-51 like kinase 1 (Ulk1), Rab9, receptor-interacting serine/thronine protein kinase 1 (Rip1), and dynamin-related protein 1 (Drp1). This complex allowed the recruitment of trans-Golgi membranes associated with Rab9 to damaged mitochondria through S179 phosphorylation of Rab9 by Ulk1 and S616 phosphorylation of Drp1 by Rip1. Knockin of Rab9 (S179A) abolished mitophagy and exacerbated the injury in response to myocardial ischemia, without affecting conventional autophagy. Mitophagy mediated through the Ulk1/Rab9/Rip1/Drp1 pathway protected the heart against ischemia by maintaining healthy mitochondria.
Authors
Toshiro Saito, Jihoon Nah, Shin-ichi Oka, Risa Mukai, Yoshiya Monden, Yasuhiro Maejima, Yoshiyuki Ikeda, Sebastiano Sciarretta, Tong Liu, Hong Li, Erdene Baljinnyam, Diego Fraidenraich, Luke Fritzky, Peiyong Zhai, Shizuko Ichinose, Mitsuaki Isobe, Chiao-Po Hsu, Mondira Kundu, Junichi Sadoshima
Graphical abstract



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)