Review
Abstract
GLP-1 receptor agonist (GLP-1RA) medications have transformed the treatment of type 2 diabetes (T2D) and obesity, with robust evidence for cardiovascular and renal benefits. Nevertheless, GLP-1RA therapy is associated with a pattern of adverse events affecting their safety and tolerability. Here, we delineate mechanisms potentially leading to adverse responses to GLP-1RAs, describe the impact of side effects on treatment persistence, discuss potential mitigation strategies, and identify areas requiring further studies. Concerns that GLP-1RAs raise the risk for acute pancreatitis and pancreatic cancer have been dispelled by long-term clinical trials. However, GLP-1RAs may confer an increased risk for thyroid cancer. Sight-threatening eye complications resulting from rapid reductions in glycemia may be avoided by retinal screening and ophthalmologic treatment before GLP-1RA initiation. The slowing of gastric emptying with GLP-1RA treatment increases the propensity for retained gastric contents, which could increase the risk of aspiration during upper gastrointestinal endoscopy or general anesthesia. These risks may, however, be elevated in individuals with long-standing T2D even in the absence of GLP-1RA treatment. Improved pharmacovigilance and a more standardized, quantitative assessment of adverse events in clinical trials, particularly in the assessment of gastrointestinal symptoms, would facilitate definition of the benefit-risk relationship for individual medications and indications.
Authors
Ryan J. Jalleh, Nicholas J. Talley, Michael Horowitz, Michael A. Nauck
Abstract
The incretin hormone glucagon-like peptide-1 (GLP-1) exerts potent effects on glucose metabolism, prompting the development of therapeutic strategies that enhance activity of the GLP-1 receptor (GLP-1R) pathway. Inhibitors of dipeptidyl peptidase 4 (DPP-4) prolong the half-life of endogenous GLP-1 and typically achieve reductions in HbA1c of 0.5%–0.8%. However, large-scale cardiovascular (CV) outcomes trials (CVOTs) with DPP-4 inhibitors demonstrated CV safety but did not show a reduction in CV events. A second incretin-based therapeutic approach was the development of GLP-1R agonists (GLP-1RAs). Various GLP-1RAs, including liraglutide, semaglutide, and dulaglutide, demonstrated a reduction in CV outcomes in large CVOTs. Initially, these medications were only available as injectable agents for subcutaneous administration, but recent technological advancements have enabled the development of orally available GLP-1RAs. A third incretin-based approach is tirzepatide, a dual agonist of GLP-1R and glucose-dependent insulinotropic polypeptide receptor (GIPR), which achieves greater HbA1c reduction and weight loss compared with GLP-1RAs alone. Ongoing large-scale CVOTs will determine its effects on hard cardiovascular endpoints. This Review summarizes the effects of GLP-1 and GLP-1RAs in the CV system as well as clinical data of GLP-1RAs in individuals with CV disease or high CV risk.
Authors
Florian Kahles, Andreas L. Birkenfeld, Nikolaus Marx
Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1RAs), established therapies for type 2 diabetes and obesity, are increasingly recognized for their potential in neurodegenerative diseases. Preclinical studies across diverse neurodegenerative conditions consistently demonstrate neuroprotective effects of GLP-1RAs, including reduced protein aggregation, enhanced autophagy, improved mitochondrial function, suppression of neuroinflammation, and preservation of synaptic integrity. Epidemiological analyses further suggest reduced incidence of dementia, Parkinson disease, and multiple sclerosis among long-term GLP-1RA users. Early human trials provide signals of target engagement, such as preserved cerebral glucose metabolism, altered inflammatory biomarkers, and slowed brain atrophy, although clinical outcomes to date remain mixed and trials in rarer disorders are sparse. Translation is constrained by uncertainty around optimal molecule choice, CNS penetrance, tolerability, adherence, and heterogeneity of response. Furthermore, next-generation dual and triple agonists may offer enhanced efficacy but remain untested in neurodegeneration. Conceptually, GLP-1RAs share pleiotropic effects with exercise — one of the few interventions with proven disease-modifying potential — by enhancing insulin signaling, stabilizing mitochondria, reducing inflammation, and promoting synaptic plasticity. This overlap highlights their promise as “pharmacological analogues of exercise,” and underscores the need for biomarker-driven, disease-specific trials to establish whether GLP-1RAs can deliver durable disease modification across the spectrum of neurodegenerative diseases.
Authors
Dilan Athauda, Nigel H. Greig, Wassilios G. Meissner, Thomas Foltynie, Sonia Gandhi
Abstract
Circadian clocks govern daily rhythms in cellular and physiological processes, including cell cycle, DNA repair, metabolism, and immune function, that influence cancer development and treatment response. Disruption of circadian regulators either promotes or suppresses malignancy depending on tumor type and biological context. This duality likely reflects systemic rewiring of circadian physiology and direct interactions between clock components and oncogenic pathways. These insights hold clinical relevance for the field of chronotherapy, which seeks to enhance therapeutic efficacy and minimize toxicity by aligning drug administration with circadian rhythms or by targeting elements of the molecular clock. In this Review, we highlight the promise of integrating circadian biology into precision oncology and underscore the importance of cancer type–specific investigations to harness the full therapeutic potential of chronotherapy in cancer.
Authors
Rebecca M. Mello, Selma Masri, Katja A. Lamia
Abstract
Metastatic hormone-sensitive prostate cancer (mHSPC) is a clinically and molecularly heterogeneous disease. Recent insights into the biology underlying disease presentation, volume of disease, and response to therapies are starting to point toward biomarkers to improve selection for intensified and deintensified treatment strategies. In addition, the therapeutic landscape is rapidly changing, with new biomarker-driven studies targeting genotype (e.g., BRCA or PTEN mutant) and phenotype (e.g., prostate-specific membrane antigen status) in development for mHSPC. A better understanding of tumor heterogeneity, clonal evolution, and metastatic homing in prostate cancer will hopefully inform future strategies for local and systemic disease control, personalized monitoring strategies, and improved patient outcomes.
Authors
Alice Bernard-Tessier, Himisha Beltran
Abstract
Cells release extracellular vesicles (EVs) with cargo that originates from distinct subcellular compartments, including the nucleus, cytoplasm, and plasma membrane. Given their diverse cargo, EVs play multiple roles in physiology and pathology, including in immune dysregulation and autoimmune pathogenesis. For example, EVs can act as autoantigens by transporting immunogenic molecules from the nucleus or cytoplasm, whereas EVs carrying membrane-bound MHCs from antigen-presenting cells can activate adaptive immunity by presenting self-antigens to T cells. EV-associated cytoplasmic peptidases or proteasomes contribute to immune regulation by modulating antigen processing and presentation. Moreover, EVs also drive inflammatory responses by shuttling a variety of proinflammatory molecules that sustain autoimmune responses. Intriguingly, emerging evidence indicates that EVs might contribute to autoimmune surveillance by activating cytosolic surveillance sensors, modulating immune checkpoints, regulating NK/T cell cytotoxicity, and altering macrophage and DC phagocytosis, representing an exciting and underexplored frontier in autoimmune research. By tackling critical knowledge gaps, this Review explores the emerging roles of EVs and their diverse cargo in driving autoimmune diseases, suggesting new perspectives on their potential as innovative therapeutic targets.
Authors
Yin Zhao, Xing Lyu, Xiuhua Wu, Yu Liu, Na Zhang, Wei Wei, Ming-Lin Liu
Abstract
Advances in cancer therapy have greatly extended patient survival but have also introduced a growing burden of cardiovascular toxicity that threatens long-term outcomes. These toxicities encompass a broad and often unpredictable range of clinical presentations, complicating oncologic care. Understanding how chemotherapy, targeted agents, and immune modulators impair cardiovascular function is essential for early detection, prevention, and management. Emerging insights into the cellular and molecular mechanisms, ranging from immune activation to transcriptional reprogramming and disrupted intercellular communication, underscore the complexity of cancer therapy–induced cardiac injury. Unraveling these mechanisms will be key to developing personalized, mechanism-based strategies that preserve cardiac function without compromising anticancer efficacy. As survivorship continues to improve, mitigating cardiotoxicity remains a critical priority for preserving both the quality and duration of life of patients.
Authors
Giulia Guerra, Marco Mergiotti, Hossein Ardehali, Emilio Hirsch, Alessandra Ghigo
Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have become an essential drug class for treating type 2 diabetes, offering proven benefits in glycemic control, weight reduction, and cardiovascular and renal protection. However, growing evidence of heterogeneity in GLP-1RA treatment effects highlights the potential for developing precision medicine approaches to more accurately allocate GLP-1RAs to maximize patient benefit. In this Review, we explore the evidence for treatment effect heterogeneity with GLP-1RAs, focusing on clinical and genetic factors that robustly influence established therapeutic outcomes. We also highlight the potential of recent predictive models that integrate routine clinical data with personalize treatment decisions, comparing GLP-1RA to other major type 2 diabetes drug classes. While such models have shown considerable promise in identifying optimal type 2 diabetes treatment based on glycemic response, their utility for informing treatment choice for other clinical outcomes remains largely unexplored.
Authors
Pedro Cardoso, John M. Dennis, Ewan R. Pearson
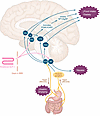
Abstract
Historically, antiobesity medications have been modestly effective at best, with side-effect profiles that limit compliance and often preclude the long-term therapy required to maintain weight loss. Recently developed therapies based on analogs of the gut hormone glucagon-like peptide-1 (GLP-1) have transformed the medical management of obesity, leading both to a degree of weight loss that rivals bariatric surgery and a reduction in morbidity and mortality associated with obesity-related complications. GLP-1 receptor agonist (GLP-1RA) therapies were developed to mimic the peripheral effects of GLP-1, but it is now well established that their efficacy in the treatment of obesity depends on reducing energy intake through their action in the central nervous system (CNS). Recent data indicate that the aversive gastrointestinal side effects of GLP-1RAs are also CNS mediated. Although a complete understanding of the neural circuits underlying GLP-1RA–induced weight loss remains elusive, a great deal has been learned in recent years. This Review summarizes proposed gut-brain and central mechanisms through which GLP-1 and its synthetic analogs regulate food intake and bodyweight.
Authors
Lisa R. Beutler
Abstract
The glucagon-like peptide-1 receptor (GLP-1R) is a class B1 G protein–coupled receptor and major therapeutic target in type 2 diabetes and obesity. Beyond its canonical role in Gαs/cAMP signaling, GLP-1R is increasingly recognized as an organizer of spatiotemporally defined signaling nanodomains, or “signalosomes.” This Review highlights our current knowledge on the mechanisms of assembly and regulation of GLP-1R signalosomes, including the involvement of biomolecular condensates formed by liquid-liquid phase separation, and the role of membrane contact sites between the endoplasmic reticulum (ER) and other organelles as key locations for GLP-1R signaling assemblies. Furthermore, we discuss existing data on the molecular composition and functional impact of two predicted GLP-1R nanodomains, one at ER–plasma membrane contact sites, where GLP-1R might interact with ion channels and transporters to influence local excitability and coordinated insulin secretion, and another at ER–mitochondria membrane contact sites, with the capacity to control lipid and calcium signaling and modulate ER and/or mitochondrial activity. We additionally discuss the role of GLP-1R posttranslational modifications as critical modulators of GLP-1R signal specification and nanodomain organization. Conceptualizing GLP-1R as a dynamic architect of spatiotemporally encoded signalosomes opens new avenues for a deeper understanding of incretin biology with the potential for identification of novel GLP-1R effectors and the development of refined therapeutic strategies for metabolic disease.
Authors
Gregory Austin, Alejandra Tomas
No posts were found with this tag.