Commentary
Abstract
Osteoarthritis (OA) is a highly prevalent and painful joint disease in desperate need of disease-modifying therapeutics. Decline in the activity of the Forkhead box O (FOXO) family of transcriptional regulators in articular chondrocytes may contribute to the development of OA. In a study in this issue of the JCI, Kurakazu et al. screened compounds for FOXO activators and discovered that the antihistamine cyproheptadine activated FOXO3 through inhibition of the histamine H1 receptor. Cyproheptadine modulated the activity of OA-relevant pathways and reduced the severity of joint damage and pain behavior in a mouse model of OA, thus showing potential for development as a disease-modifying OA drug.
Authors
Richard F. Loeser, Philip R. Coryell
Abstract
Increased activation of the NLRP3 inflammasome in immune cells, including macrophages, has been implicated in the pathogenesis of multiple chronic inflammatory diseases. Targeted depletion of macrophages has been explored as a cross-disease therapeutic strategy, but without subtype-specific markers, this strategy risks elimination of macrophages with homeostatic functions. In this study, Liu et al. identified a subpopulation of pathogenic macrophages, referred to as Toe-Macs, which are characterized by overexpression of the DNA demethylase TET3 in metabolic dysfunction–associated steatohepatitis (MASH), non–small cell lung cancer (NSCLC), and endometriosis. When induced into the disease microenvironment, Toe-Macs produced proinflammatory cytokines and chemokines. Selective elimination of Toe-Macs attenuated disease progression without any discernible side effects in mouse models of MASH and NSCLC. These findings highlight the role of Toe-Macs in the pathogenesis of chronic inflammatory diseases and provide a rationale for exploring TET3 as a therapeutic target.
Authors
Shojiro Haji, Yoshihiro Ogawa
Abstract
In acute myeloid leukemia (AML), leukemogenesis is typically driven by the sequential acquisition of distinct classes of mutations that collaborate to transform normal hematopoietic stem and progenitor cells. The founding and cooperating mutations in AML are often in signaling genes and form functional partnerships with each other, each addressing complementary aspects of malignant transformation. In this issue of the JCI, Kramer et al. elaborate on the molecular pathogenesis of AML. By using a mouse bone marrow model bearing the common AML-initiating mutations in DNA methyltransferase 3 α (DNMT3A) and nucleophosmin 1 (NPM1), the work provides further evidence for the role of the signaling orchestrator GRB2-associated–binding protein 2 (GAB2) in AML progression, positioning GAB2 as a potential therapeutic target.
Authors
Amanda Luvisotto, Lu Wang
Abstract
Celiac disease, an enteropathy driven by a maladaptive immune response to dietary gluten, is marked by increased proliferation in intestinal crypts, or crypt hyperplasia. However, it is unknown whether this phenomenon is a compensatory response to loss of villus epithelial cells or if it is driven by independent mechanisms. In this issue of the JCI, Stamnaes et al. demonstrated that in untreated celiac disease, crypt cells had increased expression of proteins involved in the IFN response, with decreased expression of fatty acid metabolism pathways. These expression patterns were recapitulated in mice treated with IFN-γ, but not mice with intestinal epithelial cell–specific knockout of the IFN-γ receptor. The findings suggest that crypt cells were reprogrammed directly by IFN-γ signaling, independent of changes to epithelial villi.
Authors
Alexa R. Weingarden
Abstract
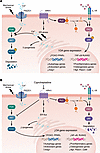
Neutrophils are key drivers of inflammation in Sweet syndrome (SS), a rare inflammatory skin disorder, but how they remain persistently activated in SS skin lesions has been unclear. In this issue of the JCI, Huang, Sati, and colleagues applied single-cell RNA-Seq and immunofluorescence to identify a subset of neutrophils in SS skin that display antigen-presenting cell–like (APC-like) features. The authors showed that when neutrophils interacted with keratinocytes, their lifespan was markedly extended, and they expressed MHC class II via activation of the serum amyloid A1/formyl peptide receptor 2 (SAA1/FPR2) signaling pathway. This, in turn, enabled T cell activation and sustained self-perpetuating inflammatory loops. These findings reveal a previously unrecognized keratinocyte-neutrophil circuit in SS and point to the SAA1/FPR2 axis as a potential target for more precise, mechanism-based therapy.
Authors
Umi Tahara, Masayuki Amagai
Abstract
Chronic limb-threatening ischemia (CLTI), the advanced stage of peripheral artery disease (PAD), remains a leading cause of morbidity and limb loss. Effective vascular regeneration strategies will require increased understanding of molecular mechanisms underlying angiogenesis. Recent evidence revealed a new role for the vascular smooth muscle cell–enriched (VSMC-enriched) long noncoding RNA (lncRNA) CARMN in endothelial angiogenesis and postischemic vascular repair. CARMN was downregulated in both human CLTI muscle tissue and murine ischemia models. In VSMCs, CARMN deficiency suppressed a specific miRNA-mediated paracrine signaling axis that regulates Hedgehog signaling. In mice, deleting CARMN caused impariment in capillary growth and blood flow recovery after limb ischemia, an effect that was reversed by restoring miR-143-3p or silencing the Hedgehog inhibitor HHIP. The identification of lncRNA-mediated crosstalk between VSMCs and endothelial cells in PAD pathophysiology reveals possible therapeutic targets for CLTI and underscores the translational potential of RNA-based strategies in ischemic vascular disease.
Authors
Shivangi Pande, George Ishak, Fahimeh Varzideh, Gaetano Santulli
Abstract
Recent advances in sequencing technologies have enabled the identification of intermediate cell states during alveolar epithelial differentiation, which expand during repair following injury and in fibrotic lungs. Although ER stress has been implicated in pulmonary fibrosis, the underlying mechanisms remain elusive. The featured study by Auyeung and colleagues looked for links between the unfolded protein response sensor inositol-requiring enzyme 1α (IRE1α), intermediate epithelial cell states, and fibrotic remodeling in the lung. They identified Regulated IRE1-Dependent Decay (RIDD) as a key effector of IRE1α signaling that drives differentiation of alveolar epithelial type 2 cells to damage-associated intermediate cells and contributes to pulmonary fibrosis, likely by degrading Fgfr2 mRNA. These findings unveil therapeutic targets and open new avenues for investigating the interplay between cellular stress responses, epithelial differentiation, and fibrotic disease.
Authors
SeungHye Han
Abstract
Initial efforts to control HIV infection include an autologous neutralizing antibody (aNAb) response. aNAbs bind Env trimers of the infecting HIV strain to neutralize virus but are not very effective at controlling HIV, as the virus quickly develops escape mutations to evade neutralization. Nevertheless, recent evidence suggests that aNAbs exert ongoing immune pressure on viral isolates in people living with HIV (PWH) treated with anti-retroviral therapy (ART) during chronic and early infection. In this issue of the JCI, McMyn et al. studied the dynamics of aNAb resistance in a cohort of 31 PWH treated with ART. Notably, a large proportion of HIV reservoir viral isolates were resistant to aNAb neutralization, which correlated with longer duration on uninterrupted ART, suggesting that selection for aNAb-resistant isolates occurs as reservoir cells containing neutralization-sensitive isolates are eliminated. aNAb resistance was not attributed to waning antibody response, which persisted for over 20 years despite viral suppression.
Authors
Nancie M. Archin
Abstract
Uniform radiation therapy (RT) de-escalation in HPV+ oropharyngeal squamous cell carcinoma (OPSCC) has underperformed in clinical trials, likely due to underlying genomic heterogeneity. In this issue of the JCI, Ho et al. evaluated genomic adjusted radiation dose (GARD), which integrates tumor gene expression with RT dose to estimate biological effect. In 191 locoregionally advanced HPV+ OPSCC patients treated with definitive RT with or without chemotherapy, GARD values varied widely, despite uniform dose delivery, and independently predicted overall survival. These data support a genomically informed framework specific for HPV+ OPSCC patients via GARD for guiding radiation dose de-escalation strategies.
Authors
Sandip K. Rath, David S. Yu
Abstract
The numbers of insulin-producing β cells in the pancreas are reduced in people with type 1 or type 2 diabetes, prompting efforts to replace these missing or lost β cells through transplant or regenerative medicine approaches. In this issue of the JCI, Wortham et al. describe a function for the deacetylase enzyme sirtuin 2 (SIRT2) in a novel pathway that acts as a brake on β cell proliferation. They show that inhibiting SIRT2 through pharmacologic or genetic approaches can induce human and mouse β cells to reenter a proliferative cell cycle. A surprising observation that remains unexplained is that the main targets of SIRT2 are mitochondrial oxidative phosphorylation (OxPhos) enzymes. It also remains unknown if and how these unanticipated acetylated OxPhos targets lead to cell-cycle entry. SIRT2 inhibitors will be a welcome addition to the growing repertoire of human β cell–regenerative drugs.
Authors
Liora S. Katz, Donald K. Scott, Andrew F. Stewart
No posts were found with this tag.



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)