Enteric Nervous System
Series edited by Rodger A. Liddle
The enteric nervous system (ENS) encompasses extrinsic and intrinsic neurons, glia, and sensory epithelial cells that are embedded throughout the gastrointestinal tract. The circuits formed by these cells are responsible for interpreting sensory information in the gut lumen in order to regulate gut motility, secretion, food intake, and immune function. The ENS communicates with the CNS in a bidirectional manner, allowing stimuli in the gut to influence mood, food intake, and other behaviors. Reviews in this series examine the mechanisms by which the ENS develops from neural crest cells, chemosensory mechanisms that allow for the detection of and response to fats and other nutrients within the gut lumen, the role of the enteric glia, regulation of ENS function by the immune system and inflammation, and the impact of surgery and the gut microbiota on ENS communication with the brain.
Articles in series
Abstract
The enteric nervous system has been studied thus far as an isolated unit. As researchers probe deeper into the function of this system, it is evident that the neural network stretches beyond enteric neurons. It is formed by both intrinsic and extrinsic neurons innervating the gut, enteric glia, and innervated sensory epithelial cells, such as enteroendocrine cells. This Review series summarizes recent knowledge on function and disease of nerves, glia, and sensory epithelial cells of the gut in eight distinctive articles. The timing and growing knowledge for each individual field calls for an appropriate term encompassing the entire system. We call this neuronal ensemble the “gut connectome” and summarize the work from a food sensory perspective.
Authors
Diego V. Bohórquez, Rodger A. Liddle
Abstract
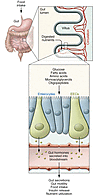
Fat is a vital macronutrient, and its intake is closely monitored by an array of molecular sensors distributed throughout the alimentary canal. In the mouth, dietary fat constituents such as mono- and diunsaturated fatty acids give rise to taste signals that stimulate food intake, in part by enhancing the production of lipid-derived endocannabinoid messengers in the gut. As fat-containing chyme enters the small intestine, it causes the formation of anorexic lipid mediators, such as oleoylethanolamide, which promote satiety. These anatomically and functionally distinct responses may contribute to the homeostatic control and, possibly, the pathological dysregulation of food intake.
Authors
Nicholas V. DiPatrizio, Daniele Piomelli
Abstract
The enteric nervous system (ENS) is sometimes called the “second brain” because of the diversity of neuronal cell types and complex, integrated circuits that permit the ENS to autonomously regulate many processes in the bowel. Mechanisms supporting ENS development are intricate, with numerous proteins, small molecules, and nutrients that affect ENS morphogenesis and mature function. Damage to the ENS or developmental defects cause vomiting, abdominal pain, constipation, growth failure, and early death. Here, we review molecular mechanisms and cellular processes that govern ENS development, identify areas in which more investigation is needed, and discuss the clinical implications of new basic research.
Authors
Marina Avetisyan, Ellen Merrick Schill, Robert O. Heuckeroth
Abstract
The enteroendocrine system is the primary sensor of ingested nutrients and is responsible for secreting an array of gut hormones, which modulate multiple physiological responses including gastrointestinal motility and secretion, glucose homeostasis, and appetite. This Review provides an up-to-date synopsis of the molecular mechanisms underlying enteroendocrine nutrient sensing and highlights our current understanding of the neuro-hormonal regulation of gut hormone secretion, including the interaction between the enteroendocrine system and the enteric nervous system. It is hoped that a deeper understanding of how these systems collectively regulate postprandial physiology will further facilitate the development of novel therapeutic strategies.
Authors
Arianna Psichas, Frank Reimann, Fiona M. Gribble
Abstract
Enteric glia are important components of the enteric nervous system (ENS) and also form an extensive network in the mucosa of the gastrointestinal (GI) tract. Initially regarded as passive support cells, it is now clear that they are actively involved as cellular integrators in the control of motility and epithelial barrier function. Enteric glia form a cellular and molecular bridge between enteric nerves, enteroendocrine cells, immune cells, and epithelial cells, depending on their location. This Review highlights the role of enteric glia in GI motility disorders and in barrier and defense functions of the gut, notably in states of inflammation. It also discusses the involvement of enteric glia in neurological diseases that involve the GI tract.
Authors
Keith A. Sharkey
Abstract
Tremendous progress has been made in characterizing the bidirectional interactions between the central nervous system, the enteric nervous system, and the gastrointestinal tract. A series of provocative preclinical studies have suggested a prominent role for the gut microbiota in these gut-brain interactions. Based on studies using rodents raised in a germ-free environment, the gut microbiota appears to influence the development of emotional behavior, stress- and pain-modulation systems, and brain neurotransmitter systems. Additionally, microbiota perturbations by probiotics and antibiotics exert modulatory effects on some of these measures in adult animals. Current evidence suggests that multiple mechanisms, including endocrine and neurocrine pathways, may be involved in gut microbiota–to–brain signaling and that the brain can in turn alter microbial composition and behavior via the autonomic nervous system. Limited information is available on how these findings may translate to healthy humans or to disease states involving the brain or the gut/brain axis. Future research needs to focus on confirming that the rodent findings are translatable to human physiology and to diseases such as irritable bowel syndrome, autism, anxiety, depression, and Parkinson’s disease.
Authors
Emeran A. Mayer, Kirsten Tillisch, Arpana Gupta
Abstract
Bariatric surgery is the most effective treatment for severe obesity, producing marked sustained weight loss with associated reduced morbidity and mortality. Roux-en-Y gastric bypass surgery (RYGBP), the most commonly performed procedure, was initially viewed as a hybrid restrictive-malabsorptive procedure. However, over the last decade, it has become apparent that alternative physiologic mechanisms underlie its beneficial effects. RYGBP-induced altered feeding behavior, including reduced appetite and changes in taste/food preferences, is now recognized as a key driver of the sustained postoperative weight loss. The brain ultimately determines feeding behavior, and here we review the mechanisms by which RYGBP may affect central appetite-regulating pathways.
Authors
Sean Manning, Andrea Pucci, Rachel L. Batterham
Abstract
Effective colonic motility involves an intricate pattern of excitatory and inhibitory neuromuscular signals that arise from the enteric neural circuitry of the colon. Recent investigations have demonstrated that inflammation leads to a variety of changes in the physiological properties of the neurons in this circuitry, including hyperexcitability of neurons at the afferent end of the peristaltic reflex, synaptic facilitation, and attenuated inhibitory neuromuscular transmission. Furthermore, links have been established between these changes and disrupted motor activity in the colon, and we now know that some of these changes persist long after recovery from inflammation. It is highly likely that inflammation-induced neuroplasticity, which is not detectable by clinical diagnostics, contributes to disrupted motility in active and quiescent inflammatory bowel disease and in functional gastrointestinal disorders.
Authors
Gary M. Mawe
Abstract
The enteric nervous system (ENS) consists of neurons and glial cells that differentiate from neural crest progenitors. During embryogenesis, development of the ENS is controlled by the interplay of neural crest cell–intrinsic factors and instructive cues from the surrounding gut mesenchyme. However, postnatal ENS development occurs in a different context, which is characterized by the presence of microbiota and an extensive immune system, suggesting an important role of these factors on enteric neural circuit formation and function. Initial reports confirm this idea while further studies in this area promise new insights into ENS physiology and pathophysiology.
Authors
Panagiotis S. Kabouridis, Vassilis Pachnis



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)