Cardiology
Series edited by Eugene Braunwald
Despite incredible progress in cardiology research, cardiovascular disease remains the leading cause of death in industrialized nations. The reviews in this series explore selected areas of cardiovascular research that show promising translational potential. Areas of interest include therapeutic antagomirs targeting cardiac microRNAs, the genetic basis of cardiomyopathies and cardiac arrhythmias, the role of chronic inflammation in atherosclerosis, S-nitrosylation in vasodilatation, as well as emerging approaches to treat heart failure, such as gene therapy, stem cell regeneration, therapeutics that restore normal calcium cycling, and interventions to reduce reperfusion injury following myocardial infarction. Advances stemming from these ongoing research efforts may soon be poised to make an impact on the clinical management of cardiovascular disease. Image credit: BSIP / Science Source.
Articles in series
Abstract
Delivery of oxygen to tissues is the primary function of the cardiovascular system. NO, a gasotransmitter that signals predominantly through protein
Authors
Saptarsi M. Haldar, Jonathan S. Stamler
Abstract
The management of cardiovascular risk through lifestyle modification and pharmacotherapy is paramount to the prevention of cardiovascular disease. Epidemiological studies have identified obesity, dyslipidemia, diabetes, and hypertension as interrelated factors that negatively affect cardiovascular health. Recently, genetic and pharmacological evidence in model systems has implicated microRNAs as dynamic modifiers of disease pathogenesis. An expanded understanding of the function of microRNAs in gene regulatory networks associated with cardiovascular risk will enable identification of novel genetic mechanisms of disease and inform the development of innovative therapeutic strategies.
Authors
Daniel Quiat, Eric N. Olson
Abstract
Genetic mutations account for a significant percentage of cardiomyopathies, which are a leading cause of congestive heart failure. In hypertrophic cardiomyopathy (HCM), cardiac output is limited by the thickened myocardium through impaired filling and outflow. Mutations in the genes encoding the thick filament components myosin heavy chain and myosin binding protein C (
Authors
Elizabeth M. McNally, Jessica R. Golbus, Megan J. Puckelwartz
Abstract
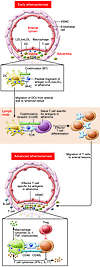
Many remarkable advances have improved our understanding of the cellular and molecular events in the pathogenesis of atherosclerosis. Chief among these is the accumulating knowledge of how the immune system contributes to all phases of atherogenesis, including well-known inflammatory reactions consequent to intimal trapping and oxidation of LDL. Advances in our understanding of the innate and adaptive responses to these events have helped to clarify the role of inflammation in atherogenesis and suggested new diagnostic modalities and novel therapeutic targets. Here we focus on recent advances in understanding how adaptive immunity affects atherogenesis.
Authors
Andrew H. Lichtman, Christoph J. Binder, Sotirios Tsimikas, Joseph L. Witztum
Abstract
Cardiovascular disease is the number one cause of mortality in the Western world. The heart responds to many cardiopathological conditions with hypertrophic growth by enlarging individual myocytes to augment cardiac pump function and decrease ventricular wall tension. Initially, such cardiac hypertrophic growth is often compensatory, but as time progresses these changes become maladaptive. Cardiac hypertrophy is the strongest predictor for the development of heart failure, arrhythmia, and sudden death. Here we discuss therapeutic avenues emerging from molecular and genetic studies of cardiovascular disease in animal models. The majority of these are based on intracellular signaling pathways considered central to pathologic cardiac remodeling and hypertrophy, which then leads to heart failure. We focus our discussion on selected therapeutic targets that have more recently emerged and have a tangible translational potential given the available pharmacologic agents that could be readily evaluated in human clinical trials.
Authors
Jop H. van Berlo, Marjorie Maillet, Jeffery D. Molkentin
Abstract
Ca2+-dependent signaling is highly regulated in cardiomyocytes and determines the force of cardiac muscle contraction. Ca2+ cycling refers to the release and reuptake of intracellular Ca2+ that drives muscle contraction and relaxation. In failing hearts, Ca2+ cycling is profoundly altered, resulting in impaired contractility and fatal cardiac arrhythmias. The key defects in Ca2+ cycling occur at the level of the sarcoplasmic reticulum (SR), a Ca2+ storage organelle in muscle. Defects in the regulation of Ca2+ cycling proteins including the ryanodine receptor 2, cardiac (RyR2)/Ca2+ release channel macromolecular complexes and the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a)/phospholamban complex contribute to heart failure. RyR2s are oxidized, nitrosylated, and PKA hyperphosphorylated, resulting in “leaky” channels in failing hearts. These leaky RyR2s contribute to depletion of Ca2+ from the SR, and the leaking Ca2+ depolarizes cardiomyocytes and triggers fatal arrhythmias. SERCA2a is downregulated and phospholamban is hypophosphorylated in failing hearts, resulting in impaired SR Ca2+ reuptake that conspires with leaky RyR2 to deplete SR Ca2+. Two new therapeutic strategies for heart failure (HF) are now being tested in clinical trials: (a) fixing the leak in RyR2 channels with a novel class of Ca2+-release channel stabilizers called Rycals and (b) increasing expression of SERCA2a to improve SR Ca2+ reuptake with viral-mediated gene therapy. There are many potential opportunities for additional mechanism-based therapeutics involving the machinery that regulates Ca2+ cycling in the heart.
Authors
Andrew R. Marks
Abstract
Advances in understanding the molecular basis of myocardial dysfunction, together with the evolution of increasingly efficient gene transfer technology, make gene-based therapy a promising treatment option for heart conditions. Cardiovascular gene therapy has benefitted from recent advancements in vector technology, design, and delivery modalities. There is a critical need to explore new therapeutic approaches in heart failure, and gene therapy has emerged as a viable alternative. Advances in understanding of the molecular basis of myocardial dysfunction, together with the development of increasingly efficient gene transfer technology, has placed heart failure within reach of gene-based therapy. The recent successful and safe completion of a phase 2 trial targeting the cardiac sarcoplasmic/endoplasmic reticulum Ca2+ ATPase pump (SERCA2a) has the potential to open a new era for gene therapy for heart failure.
Authors
Roger J. Hajjar
Abstract
Cardiovascular research is progressing on many fronts, as highlighted in the collection of Reviews in this issue of the
Authors
Eugene Braunwald
Abstract
This article discusses current understanding of myocardial biology, emphasizing the regeneration potential of the adult human heart and the mechanisms involved. In the last decade, a novel conceptual view has emerged. The heart is no longer considered a postmitotic organ, but is viewed as a self-renewing organ characterized by a resident stem cell compartment responsible for tissue homeostasis and cardiac repair following injury. Additionally, HSCs possess the ability to transdifferentiate and acquire the cardiomyocyte, vascular endothelial, and smooth muscle cell lineages. Both cardiac and hematopoietic stem cells may be used therapeutically in an attempt to reverse the devastating consequences of chronic heart failure of ischemic and nonischemic origin.
Authors
Piero Anversa, Jan Kajstura, Marcello Rota, Annarosa Leri
Abstract
Despite many innovative advances in cardiology over the past 50 years, heart disease remains a major killer. The steady progress that continues to be made in diagnostics and therapeutics is offset by the cardiovascular consequences of the growing epidemics of obesity and diabetes. Truly innovative approaches on the horizon have been greatly influenced by new insights in cardiovascular development. In particular, research in stem cell biology, the cardiomyocyte lineage, and the interactions of the myocardium and epicardium have opened the door to new approaches for healing the injured heart.
Authors
Karl Degenhardt, Manvendra K. Singh, Jonathan A. Epstein
Abstract
The abrupt cessation of effective cardiac function due to an aberrant heart rhythm can cause sudden and unexpected death at any age, a syndrome called sudden cardiac death (SCD). Annually, more than 300,000 cases of SCD occur in the United States alone, making this a major public health concern. Our current understanding of the mechanisms responsible for SCD has emerged from decades of basic science investigation into the normal electrophysiology of the heart, the molecular physiology of cardiac ion channels, fundamental cellular and tissue events associated with cardiac arrhythmias, and the molecular genetics of monogenic disorders of heart rhythm. This knowledge has helped shape the current diagnosis and treatment of inherited arrhythmia susceptibility syndromes associated with SCD and has provided a pathophysiological framework for understanding more complex conditions predisposing to this tragic event. This Review presents an overview of the molecular basis of SCD, with a focus on monogenic arrhythmia syndromes.
Authors
Alfred L. George Jr.
Abstract
The discovery of the genetic basis of inherited arrhythmias has paved the way for an improved understanding of arrhythmogenesis in a wide spectrum of life-threatening conditions. In vitro expression of mutations and transgenic animal models have been instrumental in enhancing this understanding, but the applicability of results to the human heart remains unknown. The ability to differentiate induced pluripotent stem cells (iPSs) into cardiomyocytes enables the potential to generate patient-specific myocytes, which could be used to recapitulate the features of inherited arrhythmias in the context of the patient’s genetic background. Few studies have been reported on iPS-derived myocytes obtained from patients with heritable arrhythmias, but they have demonstrated the applicability of this innovative approach to the study of inherited arrhythmias. Here we review the results achieved by iPS investigations in arrhythmogenic syndromes and discuss the existing challenges to be addressed before the use of iPS-derived myocytes can become a part of personalized management of inherited arrhythmias.
Authors
Silvia G. Priori, Carlo Napolitano, Elisa Di Pasquale, Gianluigi Condorelli
Abstract
Acute myocardial infarction (MI) is a major cause of death and disability worldwide. In patients with MI, the treatment of choice for reducing acute myocardial ischemic injury and limiting MI size is timely and effective myocardial reperfusion using either thombolytic therapy or primary percutaneous coronary intervention (PPCI). However, the process of reperfusion can itself induce cardiomyocyte death, known as myocardial reperfusion injury, for which there is still no effective therapy. A number of new therapeutic strategies currently under investigation for preventing myocardial reperfusion injury have the potential to improve clinical outcomes in patients with acute MI treated with PPCI.
Authors
Derek J. Hausenloy, Derek M. Yellon



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)