Comments for:
Citation Information: J Clin Invest. 2003;111(9):1399-1407. https://doi.org/10.1172/JCI17061.
Abstract
Cardiac hypertrophy is a common and often lethal complication of arterial hypertension. Atrial natriuretic peptide (ANP) has been postulated to exert local antihypertrophic effects in the heart. Thus, a loss of function of the ANP receptor guanylyl cyclase-A (GC-A) might contribute to the increased propensity to cardiac hypertrophy, although a causative role in vivo has not been definitively demonstrated. To test whether local ANP modulates cardiomyocyte growth, we inactivated the GC-A gene selectively in cardiomyocytes by homologous loxP/Cre-mediated recombination. Thereby we have circumvented the systemic, hypertensive phenotype associated with germline inactivation of GC-A. Mice with cardiomyocyte-restricted GC-A deletion exhibited mild cardiac hypertrophy, markedly increased mRNA expression of cardiac hypertrophy markers such as ANP (fivefold), α-skeletal actin (1.7-fold), and β-myosin heavy chain (twofold), and increased systemic circulating ANP levels. Their blood pressure was 7–10 mmHg below normal, probably because of the elevated systemic levels and endocrine actions of ANP. Furthermore, cardiac hypertrophic responses to aortic constriction were enhanced and accompanied by marked deterioration of cardiac function. This phenotype is consistent with a local function of the ANP/GC-A system to moderate the molecular program of cardiac hypertrophy.
Authors
Rita Holtwick, Martin van Eickels, Boris V. Skryabin, Hideo A. Baba, Alexander Bubikat, Frank Begrow, Michael D. Schneider, David L. Garbers, Michaela Kuhn
Untitled
Submitter: L.H. Block | lutz-henning.block@akh-wien.ac.at
University of Vienna
Published November 11, 2003
In the following I would like to respond to several queries related to our paper JCI May 2003; 111 (9) : 1339-46 by Dr. James P. Maloney.
Dr. Maloney is quite right in remarking the problem of non-specific binding when using radiolabelled ligands exposed to intact cells. It is a principal matter of debate whether one should use intact cells or isolated membranes. However, to mimic the actual clinical pathophysiological condition, the intact cells may be used as the more appropriate tool, as has been shown in numerous investigations. This was the reason why we decided to use intact cells. Having spent several years in cell biology, not only I but also my co-workers are quite experienced in radioligand binding studies. The introductory comment by Dr. Maloney is therefore somewhat superfluous, although his argument is well taken. To answer his queries specifically:
1. The various batches of 125I-VIP were labelled in the Austrian Research Centre Seibersdorf at the radiochemistry department. 125I-VIP was isolated from the reaction mixture by preparative HPLC.
2. The crucial matter of concern is the problem of non-specific binding. Like many investigators, we have chosen intact cells (pulmonary vascular smooth muscle cells). In order to perform VIP binding studies, the cells were incubated at 4°C. However, we could not completely exclude the ability of the cells to internalize radiolabelled ligand as was decribed before (Ann. NY Acad. Sci. 865: 6472, 1998). The explanation for this common observation is that the cells, even at 4° C, show some continuous membrane recycling, although the activity of membrane recycling may vary among various cell types. Thus, regardless of whether increasing concentrations of unlabelled ligand are used, one cannot exclude the possibility of internalization of radiolabelled ligand bound to corresponding receptors at the outer plasma membrane. To demonstrate this effect, we have obtained microscopic data using fluorescent labelled VIP.
3. Although we were dealing with relatively high non-specific binding, significant qualitative and quantitative changes in terms of binding capacity and binding affinity (Bmax, Kd) can be discerned. More specifically, taking into account the problem of non-specific binding at 4° C, the application of our methodology allowed us to distinguish between the binding characteristics of cell preparations of normal controls vs. PPH-patients. To reinvestigate our results, specificity of radioligand binding (same specific activity of 125IVIP) was increased by using higher concentrations of unlabelled ligand 100-fold increase as compared to 20-fold increase in our paper (see figure attached). As could be forseen, there was a change in binding parameters in the following way: as reported in our paper, Bmax was 0.57 pmol/106 cells in control cells, versus 0,89 pmol/106 cells in PPH patients and a Kd of 25.68 in normal controls corresponding to binding sites versus 1.70 nmol in PPH. This was compared to data obtained by repeated experiments using the novel conditions indicated above: Bmax 0.50 pmol/106 in controls versus 0.71 pmol/106 cells in PPH patients and a Kd of 0.55 in normal controls versus 0.61 nmol in PPH.The comparison of Kd values in our publication with reports by others using human lung tissue sections is not justified. The papers cited by Dr. Maloney compare Kds obtained from complete lung tissue (sections) including a variety of cells but not from purified pulmonary artery smooth muscle cell cultures as revealed in our publication.
To conclude, we greatly appreciate the constructive criticism by Dr. Maloney on our preliminary investigations on the role of VIP in PPH. After having repeated the experiments related to figure 6, as suggested by Dr. Maloney, we confirmed the conclusions drawn from the experiments published in our paper, i.e. an up-regulation of VIP receptors in pulmonary vascular smooth muscle cells from PPH patients as compared to controls.
In view of our experimental experience reported above, we do not think that a correction or addendum to our recent JCI-article is necessary. However, we would leave it up to you. If you decide to publish a correction or addendum, we will be happy to provide our data obtained from repeated experiments.
Sincerely yours,
L. H. Block, MD
Figure legend:
Saturation studies of 125I-VIP binding to pulmonary artery smooth muscle cells prepared from PPH (A) and control (B) patients: Each experiment was performend in triplicate. Intact cells (0,5 x 105) were incubated with increasing concentrations of 125I-VIP (0.01 to 10 nM) in the absence [total binding(TB)] and the presence of unlabeled VIP (800 nM, non-specific binding). After incubation for 1 h at 4°C, the cells were diluted 1:10 with assay buffer (4°C), and rapidly centrifuged (4500 g, 10 min, 4°C) to separate membrane-bound from free ligand. The resulting pellet was washed twice with buffer and counted in a gamma counter for 1 min. Specific binding (SB) was determined as the difference of total versus nonspecific binding (NSB). Saturation binding curves were calculated with the least square fitting method of the Origin 3.54 software (Microcal Software, MA) and Scatchard analysis was used to determine the maximal binding capacity (Bmax) and Kd values.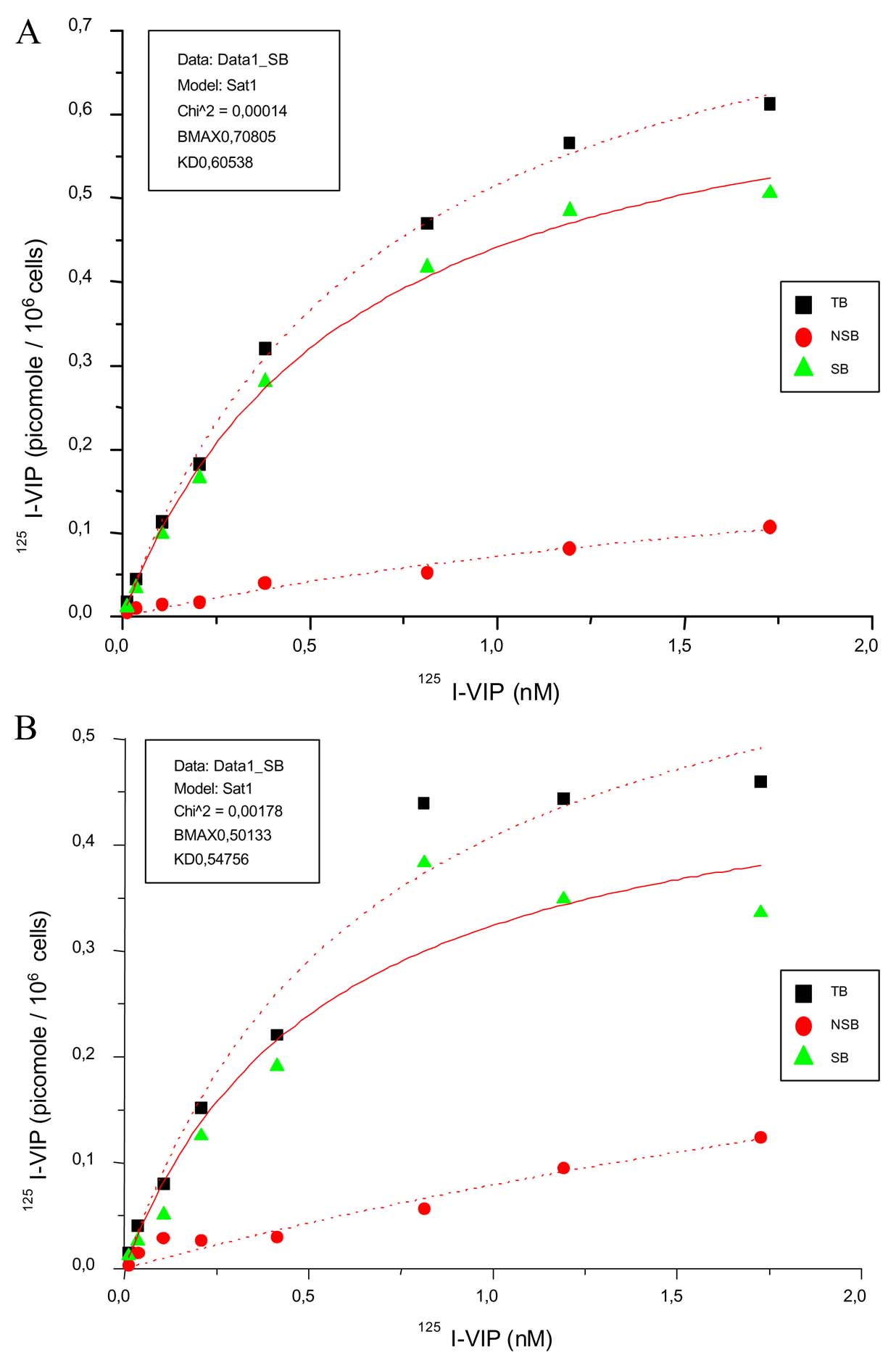
For details, see Methods of our JCI article, May 2003. 111 (9) 1339-1346.
Untitled
Submitter: James P. Maloney | jmaloney@mail.mcw.edu
Medical College of Wisconsin
Published November 11, 2003
I congratulate the authors on a fascinating body of work. However, I have concerns regarding the conclusions drawn from radioligand binding data presented in this paper. Given the importance of this paper in the field, I feel it equally important that the authors address experimental issues that limit their conclusions. It would be best if the authors submit an addendum for readers after repeating the radioligand binding experiments with attention to the comments below, and informed us readers of such updated results. One would hate to see VIP clinical trials for PH proceed based on a key paper with flawed binding data. The radioligand data in its present form is not usable.
The conclusions drawn from the 125I-VIP binding on cultured cells by the authors appear to reflect nonspecific binding (NSB), rather than specific binding (SB) of 125I-VIP. Basic principles of radioligand binding require 1) evidence of receptor saturability (that specific binding plateaus as the finite number of receptors become saturated), 2) that nonspecific binding be a minor component of total binding (TB); and that 3) binding is inhibitable by excess unlabeled, or ‘cold’ ligand (or other specific inhibitors).
Unless these criteria are met, one cannot generate meaningful Bmax, Kd, and group comparison data using Scatchard or Rosenthal analysis techniques. The data suggest what problems developed in their binding experiments.
1) Radiolabel must be highly purified (< 10% free label by TCA precipitation) and have a high specific activity. The authors did not mention whether they determined purity or specific activity of the commercially-labeled 125I-VIP used. Commercially-labeled ligands still require purification (with dialysis or gel columns) to remove free label before use. In fact, it is not unusual to have to do this weekly due to radioligand breakdown. I suspect this was one of their problems - they may have been observing the effects of free 125I in cell culture (high nonspecific binding). Excess free 125I would be expected to create high nonspecific binding, which is exactly what is reported in Figure 6. The widely disparate and extremely high levels of NSB between control PASMC (NSB at about 70%, Figure 6a) and PPH PASMC (NSB still high at 40%, Figure 6b) suggest they were perhaps done at different times with resulting different purities or specific activities of 125I-VIP. NSB should be < 20% or the basic assumptions of radioligand binding are difficult to meet. The human 123I-VIP scintigraphy results do not seem to show much free label was present, suggesting that free label removal from that different preparation (123I, not 125I) used in humans was good.
2) Excess unlabeled ligand should be at least 100-fold higher at each concentration than the 125I-VIP in order to demonstrate the level of nonspecific binding. The authors’ highest concentration of 125I-VIP was 20nm, while the corresponding blocking concentration of unlabeled VIP was only 100 nM, just a 5-fold difference. Thus, only [125I-VIP]< 1 nM would have been sufficiently blocked in their experiments. The effects of this are apparent in figure 6, where NSB is never suppressed.
3) The authors would appear to need [125I-VIP] > 18 nM, as SB never plateaus to show saturability (most evident in Figure 6a). This may not be needed if 125I-VIP is highly purified and if the appropriate blocking concentrations of unlabeled VIP are used.
Thus, the differences between PPH and control patient PASMC VIP binding may well be artifact. The authors cannot conclude that there are 2 different receptor types (p. 1344), nor are the Bmax and KD data interpretable. Their findings of KD values 100-fold higher than those in other reports of human lung1-2 supports the impact of method-related artifact. My concerns are supported by reviews of the radioligand method.3
I recommend that a JCI reviewer whose work encompasses radioligand binding have a look at these data. Alternatively, Nancy Zahniser, PhD could be approached (Department of Pharmacology, 4200 East Ninth Avenue, Box C-236, University of Colorado Health Sciences Center, 80262, Denver, CO, USA; nancy.zahniser@uchsc.edu).
Sincerely,
James P. Maloney, MD
Assistant Professor
Pulmonary and Critical Care Medicine
Cardiovascular Research Center
Medical College of Wisconsin
1. Carstairs JR, Barnes PJ. Visualization of vasoactive intestinal peptide receptors in human and guinea pig lung. J Pharmacol Exp Ther. 1986 Oct;239(1):249-55.; Leys K, Morice AH, Madonna O, Sever PS FEBS Lett. 1986 Apr 21;199(2):198-202. Autoradiographic localisation of VIP receptors in human lung)
2. Leys K, Morice AH, Madonna O, Sever PS FEBS Lett. 1986 Apr 21;199(2):198-202. Autoradiographic localisation of VIP receptors in human lung.
3. Bylund DB, et “Radioligand binding methods: practical guides and tips. AJP (Lung Cell Mol Phys)1993; 265(9):L421-429 for further help.



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)

