Advertisement
ArticleImmunology Free access | 10.1172/JCI19441
Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells
Mindi R.Walker,1 Deborah J. Kasprowicz,2 Vivian H. Gersuk,1 Angéle Bènard,2 Megan Van Landeghen,1 Jane H. Buckner,1 and Steven F. Ziegler2
1Diabetes Program and2 Immunology Program, Benaroya Research Institute at Virginia Mason, Seattle, Washington, USA
Address correspondence to: Steven F. Ziegler, Benaroya Research Institute, 1201 Ninth Avenue, Seattle, Washington 98101, USA. Phone: (206) 344-7950; Fax: (206) 223-7543; E-mail: sziegler@benaroyaresearch.org.
Find articles by R.Walker, M. in: JCI | PubMed | Google Scholar
1Diabetes Program and2 Immunology Program, Benaroya Research Institute at Virginia Mason, Seattle, Washington, USA
Address correspondence to: Steven F. Ziegler, Benaroya Research Institute, 1201 Ninth Avenue, Seattle, Washington 98101, USA. Phone: (206) 344-7950; Fax: (206) 223-7543; E-mail: sziegler@benaroyaresearch.org.
Find articles by Kasprowicz, D. in: JCI | PubMed | Google Scholar
1Diabetes Program and2 Immunology Program, Benaroya Research Institute at Virginia Mason, Seattle, Washington, USA
Address correspondence to: Steven F. Ziegler, Benaroya Research Institute, 1201 Ninth Avenue, Seattle, Washington 98101, USA. Phone: (206) 344-7950; Fax: (206) 223-7543; E-mail: sziegler@benaroyaresearch.org.
Find articles by Gersuk, V. in: JCI | PubMed | Google Scholar
1Diabetes Program and2 Immunology Program, Benaroya Research Institute at Virginia Mason, Seattle, Washington, USA
Address correspondence to: Steven F. Ziegler, Benaroya Research Institute, 1201 Ninth Avenue, Seattle, Washington 98101, USA. Phone: (206) 344-7950; Fax: (206) 223-7543; E-mail: sziegler@benaroyaresearch.org.
Find articles by Bènard, A. in: JCI | PubMed | Google Scholar
1Diabetes Program and2 Immunology Program, Benaroya Research Institute at Virginia Mason, Seattle, Washington, USA
Address correspondence to: Steven F. Ziegler, Benaroya Research Institute, 1201 Ninth Avenue, Seattle, Washington 98101, USA. Phone: (206) 344-7950; Fax: (206) 223-7543; E-mail: sziegler@benaroyaresearch.org.
Find articles by Van Landeghen, M. in: JCI | PubMed | Google Scholar
1Diabetes Program and2 Immunology Program, Benaroya Research Institute at Virginia Mason, Seattle, Washington, USA
Address correspondence to: Steven F. Ziegler, Benaroya Research Institute, 1201 Ninth Avenue, Seattle, Washington 98101, USA. Phone: (206) 344-7950; Fax: (206) 223-7543; E-mail: sziegler@benaroyaresearch.org.
Find articles by Buckner, J. in: JCI | PubMed | Google Scholar
1Diabetes Program and2 Immunology Program, Benaroya Research Institute at Virginia Mason, Seattle, Washington, USA
Address correspondence to: Steven F. Ziegler, Benaroya Research Institute, 1201 Ninth Avenue, Seattle, Washington 98101, USA. Phone: (206) 344-7950; Fax: (206) 223-7543; E-mail: sziegler@benaroyaresearch.org.
Find articles by Ziegler, S. in: JCI | PubMed | Google Scholar
Published November 1, 2003 - More info
J Clin Invest. 2003;112(9):1437–1443. https://doi.org/10.1172/JCI19441.
© 2003 The American Society for Clinical Investigation
Received: July 9, 2003; Accepted: September 16, 2003
-
Abstract
CD4+CD25+ regulatory T (TR) cells have been described in both humans and mice. In mice, TR are thymically derived, and lack of TR leads to organ-specific autoimmunity. Recently, the forkhead/winged helix transcription factor, FoxP3, has been shown to be important for the function of TR cells in mice. In this study, human TR cells were examined and, in results similar to those of studies done in mice, expression of FoxP3 was found exclusively in CD4+CD25+ T cells and correlated with the suppressive activity of these cells. In contrast to the mouse studies, activation of human CD4+CD25– T cells led to expression of FoxP3. Expression of FoxP3 in activated human CD4+CD25+ cells also correlated with suppression of proliferation by these cells in freshly isolated CD4+CD25– T cells from the same donor. This suppression was cell-contact dependent and cytokine independent. Thus, in humans, during activation of CD4+CD25– T cells in an immune response, two populations of cells may arise, effector CD4+CD25+ and regulatory CD4+CD25+ T cells, with expression of FoxP3 correlated with regulatory activity. These data also raise the possibility that a failure to generate peripheral TR cells properly may contribute to autoimmune disease and suggest a possible therapeutic role for FoxP3 in the treatment of such diseases.
-
Introduction
Immunological tolerance has developed to allow organisms to differentiate between self and nonself. T cell tolerance is achieved primarily through the elimination of potentially autoreactive clones in the thymus through a mechanism known as negative selection (1, 2). The clones that escape central tolerance in the thymus are rendered anergic in the periphery upon encounter with an antigen under suboptimal conditions (2). In spite of these two mechanisms for maintaining tolerance, autoreactive T cells can be readily detected in normal individuals. Recently, a regulatory (TR) T cell population has been identified and shown to actively suppress immune responses (3–5). These TR cells, characterized by the expression of the cell surface markers CD4 and CD25, inhibit the activation of autoreactive T cells in an antigen-specific, cell-contact–dependent manner (6). In rodents, these cells have been shown to develop in the thymus, possibly as a consequence of escape from negative selection (7, 8).
The molecular basis for the development and function of TR cells remains unclear. Work in mice with targeted mutations suggests a role for CTL-associated antigen-4 (CTLA-4) in the process, in that CTLA-4–null mice lack functional TR cells (9). However, the disease present in CTLA-4–deficient mice is much more severe than that seen in mice lacking TR cells (10, 11). Similarly, TGF-β has been implicated in the function of TR cells based on the phenotype of TGF-β–null mice (12, 13). However, work in both rodents and humans has failed to uncover a role for this cytokine in the regulatory activity of TR cells (14).
Mice carrying the X-linked scurfy mutation develop a lymphoproliferative disease similar to that seen in CTLA-4–null mice (15, 16). These mice display multiorgan autoimmune disease and lack conventional CD4+CD25+ TR cells (17, 18). FoxP3, the gene mutated in these mice, encodes a member of the forkhead/winged helix family and acts as a transcriptional repressor (19). In mice, FoxP3 has been shown to be expressed exclusively in CD4+CD25+ TR cells and is not induced upon activation of CD25– T cells. However, when FoxP3 is introduced via retrovirus or enforced transgene expression, naive CD4+CD25– T cells are converted to TR (20). Thus, in mice, FoxP3 is both necessary and sufficient for the development and function of CD4+CD25+ TR cells.
Regulatory CD4+CD25+ T cells in humans represent between 1% and 3% of total CD4+ T cells. In this report, we show that human CD4+CD25+ TR cells express FoxP3, whereas CD25– T cells do not. Expression of FoxP3 in CD4+T cells correlates with their ability to function as TR cells. Interestingly, CD4+CD25+ T cells, generated as a consequence of stimulation of CD4+CD25– human T cells, also express FoxP3 and acquire TR function. Thus, the regulation of human FoxP3 expression and function differs from that seen in mice and suggests that de novo generation of TR cells is a natural consequence of immune responses in humans.
-
Methods
Isolation of human cells. To isolate CD4+CD25+ T cells, human peripheral blood was obtained from normal healthy donors, and PBMCs were prepared by centrifugation over Ficoll-Hypaque gradients. CD4+ T cells were purified by depletion of cells expressing CD8, CD11b, CD16, CD19, CD36, and CD56 with the CD4+ No-touch T cell isolation kit (Miltenyi Biotec, Auburn, California, USA). CD4+ T cells were then labeled with 200 μl each of CyChrome conjugated anti-CD4 (RPA-T4, Pharmingen, San Diego, California, USA) and phycoerythrin-conjugated (PE-conjugated) anti-CD25 (M-A251, Pharmingen) for 20 minutes at 4°C. Cells were washed, and the highest 1–2% of CD25+ or CD25– cells were sorted via a FACSVantage (Becton Dickinson, San Jose, California, USA). Cells with high forward scatter were excluded in order to eliminate activated cells. 2 × 108 PBMCs typically yielded 2 × 105 CD25+ cells with greater than 99% purity. Accessory cells were obtained by isolating the positive fraction of the CD4+ No-touch magnetic sort after depleting CD8+ T cells with CD8 microbeads (Miltenyi Biotec). Accessory cells were irradiated with 5,000 rads. Human PBMCs were obtained after informed consent in accordance with procedures approved by the human ethics committee of the Benaroya Research Institute.
Cell stimulation assays. CD4+CD25+, CD4+CD25– (2.5 × 103 per well), or both (2.5 × 103 each per well) were cultured with irradiated accessory cells (2.5 × 104 per well). For polyclonal activation, cells were cultured with 5 μg/ml soluble anti-CD3 (UCHT1; Pharmingen) and 5 μg/ml soluble anti-CD28 (CD28.2; Pharmingen). The ability of CD25+ cells to suppress proliferation of CD25– cells was determined by 3H thymidine incorporation. For thymidine incorporation assays, half of the culture supernatant was removed (100 μl), then 1 μCi 3H thymidine was added during the final 16 hours of a 6- to 7-day assay, and proliferation was measured by scintillation counting.
Generation of CD25+ regulatory cells. In order to generate CD25+ regulatory cells from CD25– cells, CD4+ cells were isolated from normal blood as described above, and then CD25– cells were isolated by negative selection with CD25 microbeads (Miltenyi Biotec). Purity was determined to be greater than 99% CD25–, and cells were activated with 5 μg/ml plate-bound anti-CD3 (UCHT1; Pharmingen) and 1 μg/ml soluble anti-CD28. Cells were removed from the plate-bound antibody after 24 hours. Cells were cultured for 3 or 10 days and sorted based on expression of CD25 and Annexin V via a FACSVantage (Becton Dickinson). Annexin V staining was performed at 37°C in the dark by adding 15 μl of FITC-conjugated Annexin V to cells resuspended in 500-μl annexin binding buffer (10 mM HEPES/NaOH (pH7.4) 140 mM NaCl, 2.5 mM CaCl2). Cells were then Western blotted or assayed for suppressive activity by culturing, CD25+AnnexinV– (2.5 × 103 per well) or CD25–AnnexinV– (2.5 × 103 per well), or both, CD25+AnnexinV–/CD25–AnnexinV– at a 1:1 ratio (2.5 × 103 per well, each). For the suppression assay, cells were activated with 5 μg/ml each soluble anti-CD3 and anti-CD28 along with T cell–depleted accessory cells. Proliferation was measured by 3H thymidine incorporation. During the final 16 hours of a 5- to 6-day assay, 3H-thymidine was added and proliferation was measured by scintillation counting. For transwell experiments, cells were cultured in 24-well plates, with or without a 4-μm transwell separating CD4+CD25+ (50,000 cells per well) cells from CD4+CD25– (50,000 cells per well). To test the dependence of suppression on cytokines, 10 μg/ ml anti-IL-10 (JES3-19F1, Pharmingen), anti-TGF-B1,2,3 (1D11; R&D Systems, Minneapolis, Minnesota, USA) or an isotype-matched control (R35-95; MOPC-21, Pharmingen) were added to the suppression assay. The ability of these Abs to neutralize IL-10 and TGF-β at this concentration was determined in separate experiments. In these experiments, TGF-β caused reduced proliferation of CD4+ peripheral blood leukocyte (PBL) and IL-10 prevented the upregulation of MHC class II on T cells.
Analysis of FoxP3 expression. For immunoblots, isolated T cell populations were directly lysed in Laemmli sample buffer, separated on 10% SDS-PAGE gels, and transferred to nitrocellulose filters. Filters were blocked in tris-buffered saline with 0.1% TWEEN 20 plus 5% freeze-dried milk for 4 hours, incubated with polyclonal rabbit anti-human FoxP3 (1:2,000) overnight at 4°C in the same buffer and developed as described (19). Western blots were stripped and reprobed with extracellular signal-regulated kinase (ERK) 1 or 2 to control for loading. Control cells were 293T cells transfected with a human FoxP3 cDNA clone.
For quantitative real-time PCR (QPCR) analysis, RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, California, USA) according to the manufacturer’s instructions, and cDNA was prepared with 2.5 μM random hexamers (Applied Biosystems Inc., Foster City, California, USA). Message levels were quantified by real-time PCR using the ABI 7000 Sequence Detection System (Applied Biosystems Inc.). Amplification was carried out in a total volume of 25 μl for 40 to 50 cycles of 15 seconds at 95°C, 1 minute at 60°C, and product was detected using SYBR Green I dye (Molecular Probes Inc., Eugene, Oregon, USA). Samples were run in triplicate, and their relative expression was determined by normalizing expression of each target to GAPDH, and then comparing this normalized value to the normalized expression in a reference sample to calculate a fold-change value. Primers were designed so that amplicons spanned intron/exon boundaries to minimize amplification of genomic DNA. Primer sequences were as follows: GAPDH: 5′-CCACATCGCTCAGACACCAT-3′ and 5′-GGCAACAATATCCACTTTACCAGAGT-3′; FoxP3: 5′-GAAACAGCACATTCCCAGAGTTC-3′ and 5′-ATGGCCCAGCGGATGAG-3′.
-
Results
FoxP3 is preferentially expressed in human CD4+CD25+ cells. To examine the expression of FoxP3 in human cells, peripheral blood mononuclear cells were purified from normal donors and specific subsets isolated on the basis of cell-surface phenotype. FoxP3 expression was ascertained by QPCR and immunoblotting. Both total CD4+ and CD8+ T cells expressed FoxP3; however, CD4+ cells express higher levels of FoxP3 (data not shown). B cells and nonlymphoid cells showed no FoxP3 expression. The expression in human CD8+ T cells differs from data seen in the mouse where only the CD4+ subset expressed detectable FoxP3 (17, 18, 20). As mouse FoxP3 has been shown to be preferentially expressed in the CD4+CD25+ cells, we further divided human CD4+ T cells based on CD25 expression and examined FoxP3 expression. Since only the highest 1–3% of CD25+ expressing cells in human CD4+ cells have been shown to be regulatory, these cells were used in our assays as the CD25+ population. As shown in Figure 1, a and b, only the CD25+ subset of human CD4+ T cells expressed FoxP3 mRNA and protein. Thus, as was seen in mice, only the putative TR population expresses detectable levels of FoxP3 within human CD4+ T cells. Interestingly, human FoxP3 protein migrates as a doublet under these conditions, one species of which comigrates with the protein expressed in 293T cells transfected with a human FoxP3 cDNA clone (see “control” lanes in Figures 2 and 3). It is not clear at this point whether these two species reflect post-translational modifications of FoxP3, the products of differentially spliced mRNAs, or some other possibility.
 Figure 1
Figure 1CD4+CD25+ regulatory cells from normal human peripheral blood express FoxP3. (a) Real-time QPCR analysis of FoxP3 gene expression relative to GAPDH expression in purified CD4+, CD4+CD25– (CD25–), and CD4+CD25+ (CD25+) T cells from a range of normal donors. (b) Western blot analysis of FoxP3 in purified CD4+, CD4+CD25–, and CD4+CD25+ cells from two separate donors. (c) Proliferation and suppression of purified CD4+CD25– and CD4+CD25+ T cells stimulated with soluble anti-CD3 and anti-CD28. These data are from one experiment but are representative of eight separate experiments with a suppression range of 60–95%.
 Figure 2
Figure 2Activation of human CD4+CD25– T cells induces FoxP3 expression. Western blot analysis of FoxP3 expression in (a) freshly isolated, purified CD4+, CD4+CD25+, and CD4+CD25– T cells or CD4+CD25– T cells that have or have not (media) been activated with plate-bound anti-CD3/soluble anti-CD28 (anti-CD3/28) for 24 or 72 hours in the presence or absence of IL-2, (b) freshly isolated CD4+CD25– or CD4+CD25+ and activated CD4+CD25– PBMCs after sorting into CD4+CD25– and CD4+CD25+ T cells. (c) FACS plot showing percentage of cells in each population before sorting. Western blot analysis of FoxP3 expression in freshly isolated CD4+CD25– T cells or activated CD4+CD25– T cells either unsorted or sorted for CD25 and Annexin V staining. Cells were also gated and sorted on live cells by FCS versus SSC. Control cells were 293T cells transfected with a human FoxP3 cDNA clone. Parts a and b show results from one experiment but are representative of four separate experiments. Figure 2c shows results from one experiment.
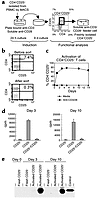 Figure 3
Figure 3CD4+CD25+ regulatory T cells can be induced by activation of human CD4+CD25– PBMCs. (a) Schematic for generation of regulatory cells from CD4+CD25– cells, including a typical FACS plot with percentages for activated CD4+CD25– cells before sorting. Cells were incubated on plate-bound Ab (Y) for 24 hours and then transferred to a new well without Ab for 9 days. (b) Dot plots showing CD4 versus CD25 staining on CD4+ PBL before and after sorting with CD25 MACS microbeads. (c) Percent of CD4+CD25+ cells over time when CD4+CD25– cells were stimulated with plate-bound anti-CD3/soluble anti-CD28 or media. (d) Proliferation and suppression of freshly isolated CD4+CD25– T cells by CD4+CD25+ cells, which were generated by activating CD4+CD25– PBMCs with plate-bound anti-CD3/soluble anti-CD28 for 3 or 10 days. (e) Western blot analysis of FoxP3 expression on day 3 or day 10 after activation of CD4+CD25– T cells. Control cells were 293T cells transfected with a human FoxP3 cDNA clone. These data are from one experiment and are representative of six separate experiments with a suppression range of 60–95%.
We next determined the function of the CD4+CD25+ T cells. For these experiments we used the CD4+CD25– and CD4+CD25+ peripheral blood T cells whose FoxP3 expression levels were shown in Figure 1 (a and b). These T cell subsets were assessed for their ability to respond to T cell receptor (TCR) stimulation, and for the ability of the CD25+ cells to suppress the in vitro activation of the CD25– cells. When cultured in the presence of feeder cells along with soluble anti-CD3 and anti-CD28, the CD4+CD25– cells responded with robust proliferation, whereas the CD4+CD25+ cells did not (Figure 1c). When the two populations were cocultured, the level of proliferation, as measured by 3H-thymidine incorporation, was dramatically reduced (Figure 1c). The level of suppression seen was correlated with the ratio of CD4+CD25–:CD4+CD25+ cells in the culture, with more CD25+ cells resulting in more suppression of CD25– cell proliferation. These results are not due to exhaustion of the resources within the culture because of the small number of cells in the culture and the fact that the addition of the same amount of CD25– cells instead of CD25+ cells does not cause suppression (see below). Similar results have been observed by other investigators for the CD4+CD25+ subset of human T cells (21–23). Thus, as has been seen in rodents, expression of FoxP3 correlated with TR activity.
TCR stimulation induces FoxP3 expression in CD4+CD25– human T cells. In addition to being a marker for TR cells, CD25 expression on CD4+ T cells is an indicator of cell activation. Specifically, stimulation of CD4+CD25– T cells through the TCR and CD28 leads to several outcomes including proliferation, cytokine production, and induction of cell-surface expression of CD25. It is, therefore, possible that some or all of the CD4+CD25+ T cells isolated from PBMCs are the result of recent activation. To assess the possibility that recently activated CD4 T cells express FoxP3, we determined FoxP3 expression in CD4+CD25– T cells stimulated through the TCR in vitro. CD4+CD25– T cells were purified from peripheral blood and stimulated with plate-bound anti-CD3 and soluble anti-CD28 in the presence or absence of IL-2, for 24 hours or 72 hours. At the indicated times, cells were harvested, lysed, and examined for FoxP3 expression by immunoblotting. As shown in Figure 2a, the starting population of CD4+CD25– T cells lacked detectable FoxP3 expression. However, after 24 hours of plate-bound anti-CD3/soluble anti-CD28 stimulation, FoxP3 expression was found in both cultures (± IL-2) and was considerably increased in the 72-hour cultures. To determine the nature of the FoxP3-expressing cells in these cultures, we purified CD25– and CD25+ cells from 72-hour cultures and examined FoxP3 expression. As shown in Figure 2b, only the CD25+ cells from these cultures expressed FoxP3. From these experiments we conclude that, unlike what has been described in the mouse, stimulation of CD4+CD25– T cells through the TCR results in the induction of FoxP3 expression and that this expression correlates with cell-surface expression of CD25.
We have recently shown that T cells that overexpress FoxP3 (from mice bearing a FoxP3 transgene) do not proliferate or produce cytokines upon TCR stimulation but undergo rapid apoptosis (ref. 24 and D.J. Kasprowicz and S.F. Ziegler, unpublished observations). Thus, one possible explanation for the results in Figure 2 is that FoxP3 is induced in those stimulated T cells undergoing activation-induced cell death. To ascertain whether FoxP3 expression correlated with activation-induced cell death, fresh human CD4+CD25– T cells were activated with plate-bound anti-CD3/soluble anti-CD28 for 72 hours and were either left unsorted or sorted into CD4+CD25–AnnexinV–, CD4+CD25+AnnexinV–, or CD4+CD25+AnnexinV+ (Figure 2c). FACS analysis shows that approximately 39% of the CD4+ cells are CD25+AnnexinV–, 59% CD25–AnnexinV–, 1.5% CD25+AnnexinV+, and 1% CD25–AnnexinV+. Annexin V staining was used as a measure of apoptosis. Lysates were prepared from the various populations, and FoxP3 expression was analyzed by immunoblotting. The number of CD4+CD25–AnnexinV+ cells was consistently insufficient for analysis by Western blotting. In all cases, FoxP3 expression segregated with CD25 expression and not with Annexin V staining. Therefore, in these T cell populations, FoxP3 expression did not mark cells undergoing activation-induced cell death. Therefore, in contrast to results seen in mice, FoxP3 can be expressed upon activation of CD4+CD25– T cells in humans (17, 18).
CD4+CD25+FoxP3+ T cells that arise from in vitro stimulation have TR activity. Since freshly isolated CD4+CD25+ T cells expressed FoxP3 and displayed regulatory activity, we asked whether CD25+ cells, arising from in vitro stimulation, also had suppressor cell activity. For these experiments, CD4+CD25+ and CD4+CD25– cells were isolated at days 3 and 10 of the culture as described above. At each time point, CD4+CD25– cells were freshly purified from the same donor and used in coculture suppression assays (Figure 3a). Figure 3b shows a representative dot plot of CD4+CD25– cells isolated by magnetic cell sorting (MACS). Several methods for activation of CD4+CD25– cells were employed; however, activation with plate-bound anti-CD3 and soluble anti-CD28 consistently resulted in a higher percentage of CD25+ cells after 10 days of culture (Figure 3c). Although both anti-CD3/CD28 bead-activated and plate-bound anti-CD3/soluble anti-CD28–activated CD4+CD25– cells induced FoxP3, the high signal strength of the plate-bound anti-CD3/soluble anti-CD28 was optimal for generation of T cells expressing FoxP3 (data not shown). Suppression assays were then performed with freshly isolated autologous CD4+CD25– T cells using soluble anti-CD3/CD28 along with feeder cells as has been done by others (18, 20, 21, 25). The CD4+CD25+ cells derived from CD4+CD25– cells activated with plate-bound anti-CD3/soluble anti-CD28 were able to suppress proliferation of freshly isolated CD4+CD25– cells (Figure 3d). Identical results were obtained when CD25– cells were FACS sorted instead of MACS sorted before the 10-day culture. In addition, CD25+ cells from the FACS sort were activated and found that less than 1% of these cells remained alive by day 10 after activation, thus negating the possibility that pre-existing CD25+ cells are expanding in these cultures. FoxP3 expression also correlated with suppressive activity (Figure 3e). These data demonstrate that CD4+CD25+ T cells with regulatory characteristics can arise from CD4+CD25– cells upon activation and that this regulatory activity is associated with the expression of FoxP3.
Furthermore, the suppressive activity of regulatory CD4+CD25+ generated from CD4+CD25– T cells is cell-contact dependent and cytokine independent. Separation of plate bound anti-CD3/soluble anti-CD28–activated CD4+CD25+ cells from freshly isolated CD4+CD25– cells by a transwell abrogates suppression of these cells (Figure 4a). As a control, activated-sorted CD4+CD25– cells were used in place of activated-sorted CD4+CD25+ cells. In contrast to results seen with activated-sorted CD25+ cells, in this assay, activated-sorted CD25– cells did not suppress proliferation. However, in one experiment, the CD4+CD25– cells isolated from activated cultures displayed suppressor activity and FoxP3 expression, suggesting that CD25 may not be a marker for all TR. Experiments are planned to explore this possibility. In addition, TR activity by generated CD25+ cells did not require IL-10 or TGF-β (Figure 4b). The addition of 10 μg/ml of anti–IL-10 or anti–TGF-β Ab has been shown to ablate suppressive activity of T regulatory type 1 (TR1) cells (26, 27), however, the same concentration of this Ab did not inhibit suppression by activated CD4+CD25+ cells generated from CD4+CD25– cells. These generated CD4+CD25+ regulatory cells are therefore similar to freshly isolated CD4+CD25+ TR from both humans and mice.
 Figure 4
Figure 4Activation-induced CD4+CD25+ regulatory T cells are cell-contact dependent and cytokine independent. Proliferation and suppression of freshly isolated CD4+CD25– T cells by CD4+CD25+ (a, left panel, and b) or CD4+CD25– (a, right panel) cells, which were generated by activation CD4+CD25– PBMCs with plate-bound anti-CD3/soluble anti-CD28 for 14 days. (a) CD4+CD25+ and CD4+CD25– cells from the same culture were sorted and cultured alone, together with freshly isolated CD25– cells, or separated by a transwell. Sorted cells are indicated in bold type and freshly isolated cells in normal type. (b) Cells were cultured in the presence of 10 μg/ml anti–IL-10, anti-TGF-β, or an isotype control Ab (RIgG2a). These data are from one experiment but are representative of two separate experiments.
-
Discussion
It is now apparent that a small population of CD4+ T cells, identified by the expression of CD25, have the ability to regulate immune responses. These TR cells have been found and studied in humans and rodents. However, to date, the characterization of these cells has been hampered by a lack of specific molecular markers. In mice, the forkhead/winged helix family protein FoxP3 has very recently been shown to be expressed predominantly in TR cells and to be critical for their generation and function (17, 18, 20). In addition, male mice carrying the X-linked scurfy mutation, which is a loss-of-function mutation in the FoxP3 gene, lack TR cells and die at 3 to 4 weeks of age unless these mice receive transferred CD25+ regulatory cells. Thus, in mice, the FoxP3 gene is both necessary and sufficient for TR cell generation and function.
In mice, FoxP3 expression appears to be limited to CD4+CD25+ TR cells, and expression was not induced upon activation of naive T cells despite the expression of CD25 on their cell surface (17, 18). These findings suggest that the regulation of FoxP3 and induction of TR cells differs in mice and humans. However, the differences between our studies and those using mouse TR cells concerning the expression of FoxP3 after activation of CD4+CD25– T cells may be due to differences in the T cell populations used in the respective studies. We used peripheral blood-derived T cells (potentially memory), whereas the mouse studies used T cells from either spleen or lymph node (mainly naive). These data suggest that there may be two pathways for the generation of TR cells in humans as a result of thymic selection as in mice or as a consequence of immune responses in the periphery. This would allow for regulation of autoreactivity by the thymus-derived TR cells, which are autoreactive and have self-renewing capabilities (5). It is this population in animal models that can provide protection from self-reactive T cells in adoptive transfer models of autoimmunity. In contrast, TR cells generated from the peripheral pool may either arise from a memory T cell population as a consequence of antigenic challenge or from naive T cells during the course of an immune response. Human TR cells isolated from peripheral blood are CD4+CD25+ CD45RO+ and CD45Rblow, consistent with memory T cells that have undergone multiple rounds of stimulation (25). These cells also have shorter telomere length as compared with CD4+CD25– CD45RO+ group. In addition, Taams et al. demonstrated that a T cell clone that was made anergic by activation in the absence of a professional APC has suppressive properties (25). A possible role for these cells would be to regulate antigen-specific T cells in order to contain the response and limit its spread. In this model, TR cells are not long lived and die after the initial response, allowing the differentiation of long-lived memory T cells. This model would allow for the “fencing-in” of the response while maintaining the ability to mount a recall response in case of a second challenge.
Evidence exists for in vitro generation of regulatory T cells in humans. However, the majority of these cells resemble TR1 cells rather than classical CD4+CD25+ TR. In humans, TR1 cells can be generated by stimulation with alloantigen in the presence of IL-10 and TGF-β. These TR1 cells produce high amounts of IL-10 when stimulated, and suppression by these cells is dependent on secretion of IL-10 and TGF-β (28, 29). CD4+CD25+ cells isolated from peripheral blood have also been shown to convey suppression on CD4+CD25– cells. CD4+CD25– cells that are regulatory after contact with CD4+CD25+ cells are dependent on IL-10 or TGF-β for suppression (26, 27). In addition, CD4+CD25+ cells can be expanded in vitro with either anti-CD3 and IL-2 or by repeated stimulation on immature DCs with alloantigen. Regulatory cells generated by the preceding methods are also cell-contact dependent and cytokine independent (30, 31). Evidence also exists for generation of CD4+CD25+ TR in the periphery of mice. Thorstenson and Khoruts have generated CD25+ T cells in the DO11.10 TCR transgenic mouse via oral or i.v. administration of peptide Ag and demonstrated that the resulting CD25+ T cells are anergic and have TR properties in vitro and in vivo (32).
The precise role of FoxP3 in the development and/or function of TR cells remains to be elucidated. The finding that enforced expression of FoxP3, either via retrovirus or transgene, can convert T cells to a TR phenotype suggests a determinative role. However, Khattri et al. (18) showed that a FoxP3 transgene limited to thymic expression failed to rescue sf/Y mice from scurfy disease, suggesting that constant FoxP3 expression is required for maintaining the suppressive function. These data, coupled with our findings showing that TCR stimulation of human CD4+CD25– T cells induces both FoxP3 expression and TR activity, suggest a more active role for FoxP3 in suppressor function.
We have presented data showing that the transcriptional regulator FoxP3 is expressed predominantly, if not exclusively, in TR cells and that it may serve as a master regulator of this cell population. The finding that some CD25– T cells express FoxP3 upon stimulation and demonstrate regulatory activity, suggests that TR cells may be generated in the periphery and that the CD4+CD25+ TR present in the peripheral blood may be functionally similar to those generated in vitro by activation. These findings suggest a possible therapeutic role for FoxP3 in the treatment of autoimmune diseases. The efficacy of such cell-based strategies await further understanding of the regulation and function of FoxP3 in TR cells.
-
Acknowledgments
We would like to thank K. Arumuganathan for isolation of specific subsets of cells by FACS. This work was supported by NIH grants AI48779 and AI054610 to S.F. Ziegler and an Arthritis Foundation Investigator Award to J.H. Buckner.
-
Footnotes
See the related Commentary beginning on page 1310.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: regulatory T (TR); CTL-associated antigen-4 (CTLA-4); phycoerythrin (PE); peripheral blood leukocyte (PBL); quantitative real-time PCR (QPCR); T cell receptor (TCR); magnetic cell sorting (MACS); T regulatory type 1 (TR1).
-
References
- Jameson, SC, Bevan, MJ. T-cell selection. Curr. Opin. Immunol. 1998. 10:214-219.
- Van Parijs, L, Abbas, AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998. 280:243-248.
- Maloy, KJ, Powrie, F. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2001. 2:816-822.
- Sakaguchi, S, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 2001. 182:18-32.
- Shevach, EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002. 2:389-400. View this article via: PubMed Google Scholar
- Sakaguchi, S, Sakaguchi, N, Asano, M, Itoh, M, Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995. 155:1151-1164. View this article via: PubMed Google Scholar
- Shevach, EM. Regulatory T cells in autoimmunity*. Annu. Rev. Immunol. 2000. 18:423-449.
- Itoh, M, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 1999. 162:5317-5326. View this article via: PubMed Google Scholar
- Bachmann, MF, Kohler, G, Ecabert, B, Mak, TW, Kopf, M. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J. Immunol. 1999. 163:1128-1131. View this article via: PubMed Google Scholar
- Tivol, EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995. 3:541-547.
- Waterhouse, P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995. 270:985-988.
- Shull, MM, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992. 359:693-699.
- Kulkarni, AB, Karlsson, S. Transforming growth factor-beta 1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am. J. Pathol. 1993. 143:3-9. View this article via: PubMed Google Scholar
- Piccirillo, CA, et al. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J. Exp. Med. 2002. 196:237-246.
- Lyon, MF, Peters, J, Glenister, PH, Ball, S, Wright, E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proc. Natl. Acad. Sci. U. S. A. 1990. 87:2433-2437.
- Godfrey, VL, Rouse, BT, Wilkinson, JE. Transplantation of T cell-mediated, lymphoreticular disease from the scurfy (sf) mouse. Am. J. Pathol. 1994. 145:281-286. View this article via: PubMed Google Scholar
- Fontenot, JD, Gavin, MA, Rudensky, AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003. 4:330-336.
- Khattri, R, Cox, T, Yasayko, SA, Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003. 4:337-342.
- Schubert, LA, Jeffery, E, Zhang, Y, Ramsdell, F, Ziegler, SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J. Biol. Chem. 2001. 276:37672-37679.
- Hori, S, Nomura, T, Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. 299:1057-1061.
- Baecher-Allan, C, Brown, JA, Freeman, GJ, Hafler, DA. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 2001. 167:1245-1253. View this article via: PubMed Google Scholar
- Dieckmann, D, Plottner, H, Berchtold, S, Berger, T, Schuler, G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J. Exp. Med. 2001. 193:1303-1310.
- Jonuleit, H, et al. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 2001. 193:1285-1294.
- Khattri, R, et al. The amount of scurfin protein determines peripheral T cell number and responsiveness. J. Immunol. 2001. 167:6312-6320. View this article via: PubMed Google Scholar
- Taams, LS, et al. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur. J. Immunol. 2002. 32:1621-1630.
- Jonuleit, H, et al. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J. Exp. Med. 2002. 196:255-260.
- Dieckmann, D, Bruett, CH, Ploettner, H, Lutz, MB, Schuler, G. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]. J. Exp. Med. 2002. 196:247-253.
- Chen, ZM, et al. IL-10 and TGF-β induce alloreactive CD4+CD25- T cells to acquire regulatory cell function. Blood. 2003. 101:5076-5083.
- Bacchetta, R, et al. Growth and expansion of human T regulatory type 1 cells are independent from TCR activation but require exogenous cytokines. Eur. J. Immunol. 2002. 32:2237-2245.
- Levings, MK, Sangregorio, R, Roncarolo, MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 2001. 193:1295-1302.
- Jiang, S, Camara, N, Lombardi, G, Lechler, RI. Induction of allopeptide-specific human CD4+CD25+ regulatory T cells ex-vivo. Blood. 2003. 102:2180-2186.
- Thorstenson, KM, Khoruts, A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 2001. 167:188-195. View this article via: PubMed Google Scholar
-
Version history
- Version 1 (November 1, 2003): No description



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)




