Citation Information: J Clin Invest. 2014;124(3):1382-1392. https://doi.org/10.1172/JCI72181.
Abstract
Myocardial infarction (MI) leads to cardiomyocyte death, which triggers an immune response that clears debris and restores tissue integrity. In the adult heart, the immune system facilitates scar formation, which repairs the damaged myocardium but compromises cardiac function. In neonatal mice, the heart can regenerate fully without scarring following MI; however, this regenerative capacity is lost by P7. The signals that govern neonatal heart regeneration are unknown. By comparing the immune response to MI in mice at P1 and P14, we identified differences in the magnitude and kinetics of monocyte and macrophage responses to injury. Using a cell-depletion model, we determined that heart regeneration and neoangiogenesis following MI depends on neonatal macrophages. Neonates depleted of macrophages were unable to regenerate myocardia and formed fibrotic scars, resulting in reduced cardiac function and angiogenesis. Immunophenotyping and gene expression profiling of cardiac macrophages from regenerating and nonregenerating hearts indicated that regenerative macrophages have a unique polarization phenotype and secrete numerous soluble factors that may facilitate the formation of new myocardium. Our findings suggest that macrophages provide necessary signals to drive angiogenesis and regeneration of the neonatal mouse heart. Modulating inflammation may provide a key therapeutic strategy to support heart regeneration.
Authors
Arin B. Aurora, Enzo R. Porrello, Wei Tan, Ahmed I. Mahmoud, Joseph A. Hill, Rhonda Bassel-Duby, Hesham A. Sadek, Eric N. Olson
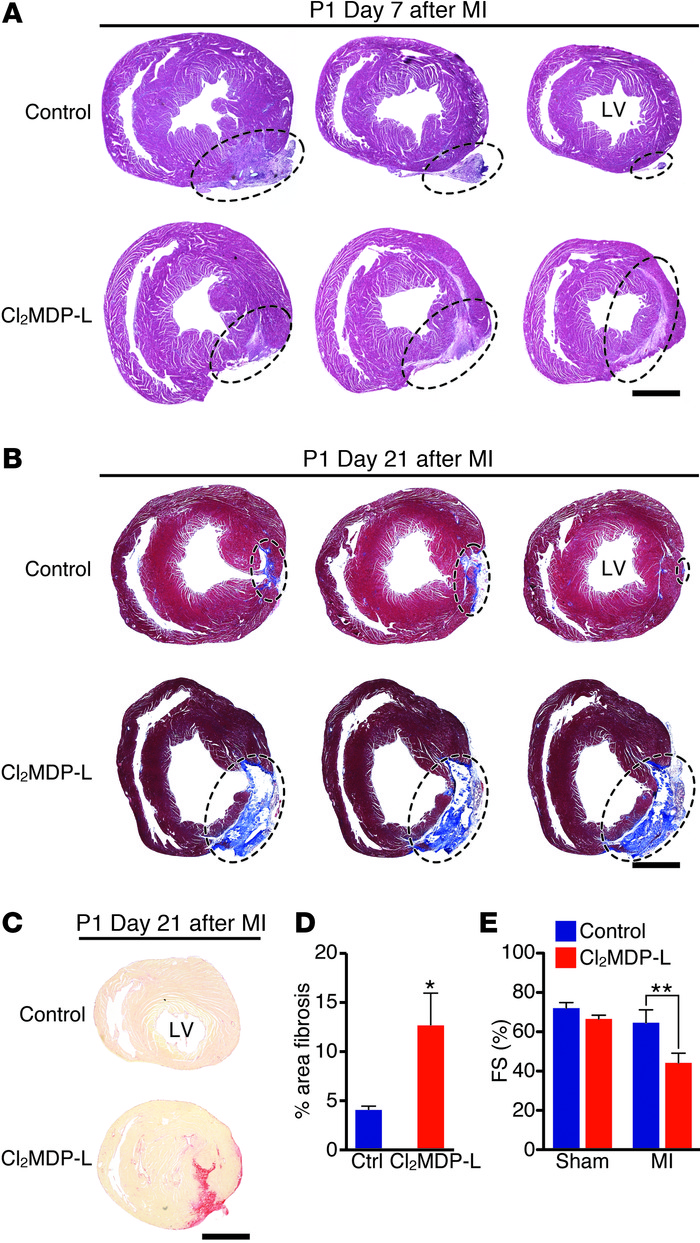
Figure 4
Depletion of monocytes/macrophages blocks heart regeneration in neonatal mice.



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)