Advertisement
Research Article Free access | 10.1172/JCI39115
Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs
Felicitas S. Boretti,1 Paul W. Buehler,2 Felice D’Agnillo,2 Katharina Kluge,1 Tony Glaus,1 Omer I. Butt,2 Yiping Jia,2 Jeroen Goede,3 Claudia P. Pereira,3 Marco Maggiorini,3 Gabriele Schoedon,3 Abdu I. Alayash,2 and Dominik J. Schaer3
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by Boretti, F. in: PubMed | Google Scholar
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by Buehler, P. in: PubMed | Google Scholar
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by D’Agnillo, F. in: PubMed | Google Scholar
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by Kluge, K. in: PubMed | Google Scholar
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by Glaus, T. in: PubMed | Google Scholar
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by Butt, O. in: PubMed | Google Scholar
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by Jia, Y. in: PubMed | Google Scholar
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by Goede, J. in: PubMed | Google Scholar
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by Pereira, C. in: PubMed | Google Scholar
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by Maggiorini, M. in: PubMed | Google Scholar
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by Schoedon, G. in: PubMed | Google Scholar
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by Alayash, A. in: PubMed | Google Scholar
1Clinic for Small Animals, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland. 2Center for Biologics Evaluation and Research, US FDA, Bethesda, Maryland, USA. 3Department of Internal Medicine, University Hospital, Zurich, Switzerland.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Find articles by Schaer, D. in: PubMed | Google Scholar
Authorship note: Felicitas S. Boretti and Paul W. Buehler contributed equally to this work.
Published July 20, 2009 - More info
J Clin Invest. 2009;119(8):2271–2280. https://doi.org/10.1172/JCI39115.
© 2009 The American Society for Clinical Investigation
Received: March 5, 2009; Accepted: May 13, 2009
Related article:
Abstract
Hemoglobin (Hb) is crucial to the function of the red blood cell. However, when it is released during intravascular hemolysis from the cell into blood plasma, it produces a state of NO depletion, oxidant stress, and vascular dysfunction, including hypertension. In their study reported in this issue of the JCI, Boretti and colleagues used canine and guinea pig models to demonstrate that pharmacological doses of glucocorticoid can increase the plasma levels of haptoglobin (Hp), the principal plasma-binding protein for free Hb (see the related article beginning on page 2271). Hp prevented Hb-induced hypertension and the generation of oxidant damage to the kidney. Neutralization of free Hb appears to be part of the downstream antiinflammatory properties of glucocorticoid.
Authors
Gregory J. Kato
-
Abstract
Release of hemoglobin (Hb) into the circulation is a central pathophysiologic event that contributes to morbidity and mortality in chronic hemolytic anemias and severe malaria. These toxicities arise from Hb-mediated vasoactivity, possibly due to NO scavenging and localized tissue oxidative processes. Currently, there is no established treatment that targets circulating extracellular Hb. Here, we assessed the role of haptoglobin (Hp), the primary scavenger of Hb in the circulation, in limiting the toxicity of cell-free Hb infusion. Using a canine model, we found that glucocorticoid stimulation of endogenous Hp synthesis prevented Hb-induced hemodynamic responses. Furthermore, guinea pigs administered exogenous Hp displayed decreased Hb-induced hypertension and oxidative toxicity to extravascular environments, such as the proximal tubules of the kidney. The ability of Hp to both attenuate hypertensive responses during Hb exposure and prevent peroxidative toxicity in extravascular compartments was dependent on Hb-Hp complex formation, which likely acts through sequestration of Hb rather than modulation of its NO- and O2-binding characteristics. Our data therefore suggest that therapies involving supplementation of endogenous Hb scavengers may be able to treat complications of acute and chronic hemolysis, as well as counter the adverse effects associated with Hb-based oxygen therapeutics.
-
Introduction
Substantial amounts of hemoglobin (Hb) are released into the circulation during rbc destruction in patients with hemolytic anemia or as a result of the destructive cycles of parasite propagation in malaria. Vasoactivity of Hb and heme-driven oxidative toxicity are causally linked to the vascular complications of hemolysis, which include pulmonary arterial hypertension, peripheral vasculopathy, stroke, and the acute chest syndrome in sickle-cell disease or cerebral malaria in the case of severe plasmodium infection (1–4). Additionally, heme-mediated toxicity is responsible for the persistent deleterious side effects of transfusion and subsequent clinical failure of Hb-based oxygen carriers (HBOCs), also known as blood substitutes (5, 6).
The toxicity of cell-free Hb is primarily attributed to ligand interactions and oxidative reactions at the heme groups (6). The strong reactivity of Hb with physiologic oxidants and subsequent oxidative modification of the protein within the extraerythrocytic environment contribute to cytotoxicity and multiple organ dysfunction and are likely involved in the chronic adverse effects associated with intravascular hemolysis (7). Our group recently identified a reproducible pattern of irreversible amino acid oxidations and heme-induced globin chain cross-link formation occurring in the presence of even low concentrations of hydrogen peroxide (H2O2), both in vitro and in vivo, providing structural evidence of these reactions (8, 9). These processes are primarily driven by the peroxidase cycle between the ferric (Fe3+) and ferryl (Fe4+) forms of Hb. Oxidative modification of non-Hb proteins and lipids, release of toxic heme iron species, as well as accumulation of irreversibly oxidized globin products accompany Hb participation in oxidative reactions and contribute to Hb oxidant and inflammatory toxicities (10). Additionally, the strong affinity of cell-free Hb for the vasodilator NO may transiently reduce NO bioavailability, with consequent endothelial dysfunction and inadequate vasopressor responses (11, 12).
The highly abundant plasma protein, haptoglobin (Hp), is the primary Hb scavenger in the circulation (13, 14). Hp irreversibly binds Hb to form a stable high-molecular-weight complex, which is subsequently cleared by the macrophage scavenger receptor, CD163 (15–17). Hb-Hp binding has developed as one of the strongest protein-protein interactions in physiologic systems, indicating that the system has evolved under exceptionally high evolutionary pressure (18). The biochemical and structural characterization of Hp is a considerable focus of research, and therapeutic implications are inferred from its potential role as a scavenger and protector against the effects of cell-free Hb (4). However, to date, only limited experimental information has been obtained on the full in vivo protective activity of Hp. Data from Hp knockout mice suggest that Hp attenuates Hb-mediated oxidative organ damage (19, 20). However, mice and most other rodents have very low baseline Hp levels, which are easily depleted upon even minor hemolysis. Consequently, it is difficult to extrapolate data from these models to ascertain the potential role of Hp in higher species.
In the current study, we evaluated 2 distinct animal models to explore the potential of Hp supplementation as a strategy to counter the intrinsic hypertensive and oxidative toxicities of free Hb. In the first model, Hp synthesis was induced in dogs by short-term glucocorticoid treatment. The dog HP gene promoter was activated by glucocorticoids, creating artificially high levels of endogenous Hp. Dogs pretreated with prednisone displayed unaltered systemic blood pressure and vascular resistance, following the application of either continuous or escalating doses of stroma-free Hb (SFHb). In the second model, exogenous Hp and SFHb were co-infused into guinea pigs. Purified human Hp prevented Hb-induced systemic hypertension and extravascular oxidative tissue injury in this animal model. We subsequently demonstrated that the protective activity of Hp is intimately related to high-affinity complex formation between circulating free Hb and its plasma scavenger protein, using chemically modified Hb with altered Hp binding properties. Our data show for the first time to our knowledge that increased Hp levels, achieved either via gene induction or exogenous administration, may play an important role in preventing complications associated with increased levels of cell-free Hb within the circulation. Complexing of SFHb with Hp, rather than extensive chemical and/or genetic modification of Hb, may additionally present a novel alternative strategy for blood transfusion therapy.
-
Results
Glucocorticoids increase the plasma Hb-binding capacity and promote intravascular compartmentalization of cell-free Hb in dogs. Glucocorticoid-responsive elements within the HP gene promoter allow high-level Hp synthesis in dogs (21, 22). To assess the influence of high endogenous Hp levels on Hb-induced physiologic responses, we treated beagle dogs with prednisone (4 mg/kg twice daily for 3 consecutive days). Prednisone treatment led to an increase in plasma Hp from 50.8 to 201.23 mg/dl (Figure 1A). Fully conscious dogs were then exposed to continuous infusion of highly purified SFHb to maintain a constant plasma heme concentration of between 100 and 150 μM over an 8-hour period. Infusion bags containing Hb solution were changed every 2–4 hours, with the percentage of oxidized Hb (Fe3+ heme) prior to changing determined to be less than 3% in all infusion bags. Size exclusion chromatography (SEC) analysis of serial plasma collections from control dogs revealed that heme (detected at 405 nm) eluted as 2 distinct peaks, representing the free Hb heterodimer at 21 minutes and the Hb bound within a larger protein (Hb-Hp complex) at 18 minutes (Figure 1C). The identity of each peak was determined using 2 independent mass spectrometry techniques, which confirmed the presence of the Hb-Hp complex (18 minutes) and Hb (21 minutes) (data not shown). In contrast, the plasma Hb-binding capacity was markedly increased in prednisone-pretreated dogs, as observed with SEC analysis of serial plasma collections (Figure 1C). Dogs pretreated with prednisone exclusively displayed the Hb-Hp complex (18 minutes) peak in plasma. The high plasma Hb-binding capacity was sustained over the entire 8-hour infusion period, and no free Hb was detected with SEC at any time point. Accordingly, Hb was rapidly observed in the urine of control dogs, while prednisone-pretreated animals remained non-hemoglobinuric over the course of the study (Figure 1B, top panel). Hb α– and β–globin chains were unequivocally detected in the dark brown urine of control dogs by MALDI–mass spectrometry (MALDI-MS) analysis, while no Hb subunits were identified in the urine of prednisone-pretreated animals sampled over 8 hours of Hb infusion (Figure 1B, bottom panel).
 Figure 1
Figure 1Increased plasma-binding capacity and intravascular retention of Hb, resulting from prednisone treatment in dogs. (A) A 4-fold increase in Hp measured in plasma after 3 days of prednisone dosing (4 mg/kg twice daily) and immediately prior to Hb infusion is depicted (data are presented as mean ± SEM). (B) Hemoglobinuria in control animals (top left panel) and, conversely, absences of hemoglobinuria in prednisone-treated animals (top right panel). High-mass MALDI-MS spectra of urines are shown (bottom panel). The control animal sample is the top spectra; the prednisone-treated animal sample is the bottom spectra. Monoisotopic ion masses of α– and β–globin chains are observed at m/z ratios of 15 and 16 kDa, respectively (top). A third ion is evident at m/z of approximately 30 kDa, suggesting the presence of a cross-linked protein species. (C) Size exclusion chromatograms of plasma collected from control (left) and prednisone-treated dogs (right). The red dotted lines signify preinfusion Hb heterodimer eluting at 21 minutes. The Hb-Hp complex is evident in the plasma of control dogs eluting at 18 minutes, with an increasing fraction of non-complexed Hb heterodimer eluting at 21 minutes over the 8-hour period. Conversely, no free Hb is observed in the plasma of prednisone-treated dogs, and only the Hb-Hp complex eluting at 18 minutes is observed over the 8-hour time course.
Glucocorticoid pretreatment prevents free Hb-mediated hypertension. We speculated that Hp-mediated intravascular Hb compartmentalization prevents Hb extravasation or limits its proximity to NO released into the vascular lumen, thereby minimizing the hypertensive response to cell-free Hb. To test this hypothesis, we performed 2 independent studies in our canine model. In the first study, the effects of a constant plasma level of extracellular Hb were examined over an 8-hour period in conscious dogs. After a bolus administration of Hb to achieve a plasma level of approximately 100–150 μM heme, the Hb concentration was maintained at a constant level by continuous infusion, and blood pressure was intermittently measured by tail artery oscillography (Figure 2A). The AUC for plasma heme concentrations over time did not differ between control and prednisone-treated animals. A significant and sustained increase in mean arterial blood pressure (MAP) of more than 20%–25% (n = 6) was observed in control dogs but not glucocorticoid pretreated animals (n = 4). However, when the infusion rate was deliberately increased until the plasma Hb-binding capacity became saturated and free Hb appeared in urine, prednisone-treated animals developed a markedly hypertensive response. Our data therefore indicate that the prednisone-mediated protection can be overcome by saturating the high plasma Hb-binding capacity in these animals (n = 2; data not shown).
 Figure 2
Figure 2Glucocorticoid pretreatment prevents free Hb-mediated hypertension. (A) The plasma Hb levels of conscious dogs were maintained at a concentration of approximately 100–150 μM (heme), with an initial bolus dose followed by constant infusion (top panel). The AUC for plasma heme concentrations over time did not differ between control (open circles, white bars) and prednisone-treated animals (filled circles, black bars). Blood pressure was monitored intermittently using tail artery oscillography over the 8-hour infusion period (bottom panel). A significant increase in MAP of more than 20%–25% (n = 6) was observed in control dogs but not in animals pretreated with prednisone (n = 4). The total volume of 0.9% NaCl plus Hb administered to dogs in each group was less than 5% of the total blood volume per hour. (B) Plasma heme concentration levels of anesthetized dogs were maintained with a Hb infusion protocol of 30 ml/h (0–30 minutes), 60 ml/h (30–60 minutes), 90 ml/h (60–90 minutes), and 120 ml/h (90–120 minutes) (top panel). The incremental increases in Hb did not differ between groups. The increase in MAP with elevated plasma heme concentrations is shown, with divergence between the 2 groups occurring at 60 minutes and corresponding to a heme plasma concentration of approximately 100–150 μM. The AUC0–120min for MAP was 5-fold greater in control (n = 5), compared with prednisone-treated animals (n = 5). Data are presented as mean ± SEM.
In the second study, we assessed the hemodynamic response to escalating plasma Hb concentrations in anesthetized dogs. A near-linear increase in plasma heme, from baseline to approximately 300 μM, was achieved via an incremental increase in the Hb infusion rate, as shown in Figure 2B. Control dogs showed a similar escalation in MAP as observed in the previous study at plasma Hb concentrations of more than 100 μM but not prednisone-pretreated dogs (n = 5). In both experimental groups, the cardiac output was not significantly altered during Hb infusion. Consistent with the well-characterized vasoconstrictive activity of cell-free Hb, the MAP increase in control dogs was associated with a roughly proportional increase in systemic vascular resistance ([SVR] increase of 29.8%, P < 0.05; Table 1). Conversely, Hb infusion did not significantly affect the SVR in prednisone-treated animals. Baseline MAP and SVR values were comparable between the 2 groups.
Hp treatment prevents Hb-induced hypertension in guinea pigs. Guinea pigs are uniquely similar to humans in circulating and tissue antioxidant status, thus making them a useful species to evaluate the vascular and extravascular effects of Hb. Conscious guinea pigs were exchange transfused at 30% of their blood volume over 5 minutes with either Hetastarch (HES), Hb, or Hb-Hp solutions. The Hb transfusions were calculated to result in peak plasma heme concentration of approximately 300 μM, with the intention to explore the possible effect of pharmacologic Hp in a condition comparable to the most severe Hb exposures observed in our dog studies. While the transfusion of HES did not affect blood pressure values monitored for 1 hour after exchange transfusion, transfusion of free Hb resulted in a rapid and strong increase in blood pressure. Concurrent treatment with Hp decreased the maximum Hb-induced MAP response (measured between 10–20 minutes after starting transfusion) from a 43% ± 14.3 % increase down to a 12.4% ± 2.7 % increase (P < 0.01) (Figure 3A). SEC analysis of plasma from animals treated with Hp confirmed that in the circulation Hb was completely bound within the large-molecular-size Hb-Hp complex. In contrast, without concurrent Hp treatment, heme elutes exclusively as free heterodimeric Hb — reflecting the negligible Hp plasma concentrations in guinea pigs (Figure 3B). In comparison with the effect observed with endogenous Hp enhancement, the co-infusion of Hp resulted in a markedly slower disappearance of Hb from plasma and complete prevention of hemoglobinuria (Figure 3, C and D). The maximum plasma concentrations of heme measured at the end of exchange transfusion were determined as 332 ± 54 μM (Hb group) and 362 ± 67 μM (Hb-Hp group), respectively. At the 4-hour blood sampling time point, heme concentrations were negligible in Hb-transfused animals. Conversely, circulating heme concentrations in Hb-Hp transfused animals remained high at 4 hours (226 ± 28 μM) and 24 hours (166 ± 16 μM). Pharmacokinetic estimates suggest an approximate 20-fold increase in the circulating half-life of Hb (0.5–1 hour) observed in our experiments (data not shown). Therefore, while formation of the large Hb-Hp complexes prolonged Hb’s circulation time, it prevented Hb from extravasation and thereby protected vulnerable organ parenchyma from contact with Hb. In the absence of Hp, free Hb rapidly leaked from plasma into the glomerular filtrate, in which it contacts the renal parenchyma. After infusion of free Hb without Hp, the total Hb concentration (as heme) in guinea pig urine was 498 ± 140 μM (1–3 ml/2 hour collection; n = 6).
 Figure 3
Figure 3Hp blunts the blood pressure response and prevents hemoglobinuria in a guinea pig model of hemolysis. (A) The MAP response (± SEM; n = 5) in animals before, during, and after exchange transfusion with HES (gray circles), Hb (open circles), or Hb-Hp (1:1) (black circles). The red bar represents the 5-minute time period of the exchange transfusion procedure (ET). (B) A representative size exclusion chromatograph at 405 nm from plasma collected after Hb (19.8 minutes) and Hb-Hp (14.7 minutes) administration. Red lines indicate preinfusion Hb and Hb-Hp elution at 19.8 minutes and 14.7 minutes, respectively. (C) Plasma total heme concentrations before and after exchange transfusion (indicated by an arrowhead). Urine total heme concentrations (4-hour collection) are shown in the graph on the right (mean ± SEM; n.d., not detected). (D) Guinea pig urine collected over a 4-hour time period following exchange transfusion with either free Hb (left) or Hb-Hp (right).
Hp treatment limits extravascular Hb peroxidative activity and oxidative tissue damage. To examine the potential effect of pharmacologic Hb administration on limiting heme iron exposure to vulnerable organs and related oxidative tissue damage, we have assessed non-heme iron and 4-hydroxynonenal–modified (4-HNE–modified) protein immunoreactivity in kidneys of the exchange-transfused animals, as a measure of peroxidative toxicity (23, 24). At 24 hours after transfusion, no Perls-detectable Fe3+ iron was observed in HES control guinea pigs (data not shown). Extensive deposition of Fe3+ iron was observed in proximal tubules but not glomeruli or medullary regions of Hb-transfused guinea pigs (Figure 4, A and B). In contrast, guinea pigs transfused with both Hb and Hp displayed no Perls-detectable Fe3+ iron. In Hb-transfused but not Hb-Hp–transfused guinea pigs, we noted an increase in 4-HNE immunoreactivity. The staining pattern demonstrated diffuse intensity, with sites of 4-HNE reactivity identified in the cortical tubular epithelial cells (Figure 4, A and B). These data illustrate the potential tissue-protective effect of early Hp administration following acute Hb exposure and are consistent with earlier studies demonstrating prevention of hemoglobinuria in actively hemolyzing rabbits (25).
 Figure 4
Figure 4Hp prevents extravascular Hb oxidative processes and oxidative tissue damage. (A) Representative bright-field images show Perls-stained (iron) kidney sections with DAB intensification and corresponding renal 4-HNE immunohistochemistry (tissue protein-bound 4-HNE) from Hb-exposed guinea pigs in the absence or presence of Hp (original magnification, ×100 [top panels]; ×400 [bottom panels]). Greater staining intensity for both iron and 4-HNE is evident at 24 hours after Hb exposure in non-Hp–treated animals. Scale bars: 200 μm (top panels); 20 μm (bottom panels). (B) Quantitative digital image analysis of total staining intensity of 30 random kidney section images from 3 Hb and 3 Hb-Hp–treated animals (mean ± SEM). (C) UV-visible spectra of Hb in urine collected 2 hours after free Hb transfusion. Each line represents the data of 1 individual animal. The derived concentrations of heme species are shown in the right panel as mean ± SEM (n = 5). (D) MALDI-MS analysis of oxidation-induced Hb globin chain cross-linking in guinea pig urine. Representative spectra of urine from Hb-transfused (left panel) and Hb-Hp–transfused animals (right panel) are shown. While no major signal of non-modified Hb can be detected at the expected m/z of human Hb α– and β–globin chains, peaks representing extensively adducted and cross-linked globin chains can be observed at m/z 23.9, 34.3, and 44.3 kDa. None of these masses appears in the spectra of Hb-Hp–treated animals.
To understand potential in vivo mechanisms leading to iron deposition and oxidative damage in the kidney of Hb-exposed animals, we evaluated the redox state of urine-excreted Hb as well as markers of oxidative globin modifications. The visible spectra of Hb displayed increased absorbance between 630 and 700 nm (Figure 4C), indicating heme iron oxidation. The urinary compositions of heme in the ferrous, Fe3+, and hemichrome forms were 303 ± 91, 175 ± 58, and 19 ± 5 μM, respectively. Stable globin cross-linking products are evident in high-mass MALDI-MS spectra of Hb in urine, indicating that globin chain interactions are consistent with the α-α covalent cross-links previously observed in oxidant-exposed Hb (10) (Figure 4D). No altered globin products could be detected in the Hb-Hp–treated animals. Thus heme-Hb redox cycling within the kidney is a likely source of iron deposition and oxidative tissue damage and is effectively prevented by intravascular sequestration of Hb by Hp administration.
High-affinity Hb-Hp interactions are essential for Hp protection against Hb-induced hypertension. To ascertain whether the protective activity of Hp is directly related to its role as a high-affinity Hb binding protein, we examined whether Hp modulates the hypertensive response induced by Bis(3,5-dibromosalicyl)fumarate-cross-linked human Hb (αα-DBBF). We previously showed that αα-DBBF — a tetrameric human Hb chemically cross-linked between α–globin chain Lys99 residues — displays markedly reduced binding affinity to Hp and does not form a stable complex (26). SEC analysis of plasma samples obtained during continuous αα-DBBF infusion into prednisone-treated dogs revealed that αα-DBBF elutes in close proximity with heterodimeric Hb and therefore was not bound within a larger protein complex in the circulation (Figure 5, A and B), despite increased Hp levels (previously shown in Figure 1A). In contrast to the situation with Hb infusion, elevated Hp levels in prednisone-treated animals did not protect against αα-DBBF–induced hypertension (Figure 6A). Similarly, in guinea pigs, we did not observe attenuation of αα-DBBF–induced hypertension by human Hp following dose-escalation studies (Figure 6B).
 Figure 5
Figure 5Absence of high-affinity Hb-Hp interactions with αα–cross-linked Hb in vivo. (A) Representative chromatograms of αα–cross-linked Hb (αα-DBBF) in plasma from prednisone-treated dogs (blue line), the Hb-Hp complex in plasma from prednisone-treated dogs (red line), Hb and the Hb-Hp complex in plasma from control dogs (solid pink), and standard noninfused Hb (black dashed line). (B) Size exclusion chromatographs of plasma over a time course following αα–cross-linked Hb administration to prednisone-treated dogs. The red dotted line represents the chromatograph of heterodimeric Hb standard eluting similarly to αα–cross-linked Hb in the plasma of prednisone-treated (high Hp) dogs. No large-molecular-size protein complex containing heme could be detected at any time point.
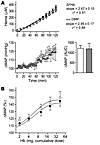 Figure 6
Figure 6Absence of high-affinity Hb-Hp interactions with αα–cross-linked Hb (αα-DBBF) leads to hypertension, despite high Hp availability. (A) Plasma heme concentrations of anesthetized dogs with a Hb and αα–cross-linked Hb (αα-DBBF) were maintained with an infusion protocol of 30 ml/h (0–30 minutes), 50 ml/h (30–60 minutes), 90 ml/h (60–90 minutes), and 120 ml/h (90–120 minutes) in control (open circles, Hb) and prednisone-treated dogs (black circles, αα-DBBF) (top panel). MAP responses to Hb (open circles) in control dogs (low Hp) and to αα–cross-linked Hb (gray circles) in prednisone-treated (high Hp) dogs (bottom panel). The AUC0–120 min for MAP was similar for both groups. Data are presented as mean ± SEM (n = 5). (B) The blood pressure response of conscious guinea pigs to dose escalation with αα–cross-linked Hb in HES (open circles) or Hp (filled circles) at a 1:1 ratio (Hb to HES or Hp). Data are presented as mean ± SEM (n = 6).
Effects of Hp on Hb biophysical properties and ligand interactions. The ligand-binding and autoxidative properties of Hb alone and Hb bound to Hp were compared to explain our in vivo results. The physical properties of Hb were comparable to those of Hb-Hp, consistent with previously published observations (9). The NO binding/oxidation rate constant of Hb versus that of the Hb-Hp complex was similar, with rate constants of 18.76 ± 1.24 μM–1 s–1 and 15.91 ± 1.49 μM–1 s–1, respectively (Figure 7A). These data suggest that blunting of the Hb-induced hypertensive response by Hp occurs via a mechanism(s) other than diminished intravascular NO inactivation. The oxygen equilibrium of Hb was left shifted in the presence of Hp from a P50 of 13.49 ± 0.315 mmHg to 9.26 ± 0.11 mmHg (Figure 7B). The cooperativity of oxygen binding (Hill number) was considerably reduced, as expected for a dimeric Hb constrained by Hp, from 2.41 ± 0.10 (Hb) to 1.44 ± 0.16 (Hb-Hp). However, these data suggest that dimeric Hb retains the ability to bind and release oxygen, similar to certain chemically stabilized HBOCs (6). The in vitro autoxidation rates assessed at 37°C over 6 hours for Hb and Hb bound to Hp were 4.165 × 10–2 h–1 and 3.498 × 10–2 h–1, respectively. These data were consistent with in vivo results demonstrating negligible Fe3+ Hb accumulation in the plasma of control and prednisone-treated dogs (Figure 7C). Therefore, nearly all the circulating heme was in the ferrous form (Figure 7D), which reacts avidly with NO, unlike Fe3+ heme. Increased Fe3+ heme formation does therefore not account for the blunted hypertensive response in prednisone-treated animals.
 Figure 7
Figure 7Effects of Hp on biophysical properties and ligand interactions of Hb. (A) Stopped-flow rapid mixing kinetic rates (k, s–1) of NO dioxygenation reactions with Hb (black circles) and the Hb-Hp complex (red circles). (B). The oxygen equilibrium curves of Hb (black line) and the Hb-Hp complex (red line). (C) Autoxidation at 37°C of Hb (black lines) and the Hb-Hp complex (red lines) over 6 hours. (D) Similarly, the autoxidation of Hb in plasma of control dogs, and autoxidation of the Hb-Hp complex as well as αα-DBBF in plasma of prednisone-treated dogs over 8 hours following the start of infusion. The white bars represent heme as Fe2+, and black bars represent heme as Fe3+. Data are presented as mean ± SEM (n = 5).
-
Discussion
Recent research focusing on the effects of intravascular free Hb on NO bioavailability led to the speculation that sequestration and compartmentalization of Hb by Hp may attenuate the intrinsic hypertensive activity of Hb (4, 27). Furthermore, the potential of manipulating the physiologic functions of Hp and/or its pharmacologic application to counter the vasoconstrictive and oxidative toxicities of intravascular free Hb has not been experimentally investigated to date. We have recently demonstrated that Hp site specifically binds and protects Hb in the presence of oxidants, such as H2O2 (9). Here, we conclusively show that Hp complex formation is effective in suppressing Hb-mediated hemodynamic imbalance as well as heme-mediated oxidative toxicity in vivo.
To better understand the role of Hb-Hp complex in ameliorating vascular toxicity, we infused free Hb in 2 different animal models to mimic intravascular hemolysis. In the course of these investigations, we made the following observations: (a) pharmacological induction of endogenous Hp synthesis or application of purified Hp results in retention of infused Hb within the intravascular compartment; (b) Hp blunts the systemic hypertensive response to cell-free Hb infusion through high-affinity complex formation; and (c) intravascular retention of Hb by Hp prevents peroxidation-related toxicity in extravascular compartments such as the kidney.
The canine model was recently proposed for the study of Hb-induced acute vascular toxicity (28). In comparison to most laboratory animals that display very low baseline Hp levels, dogs contain Hp plasma concentrations within the range found in human plasma (29). Although speculative at this point, the similar, high Hp concentrations found in dog and human may be related to a similar extent of mechanical intravascular hemolysis in the 2 species and thus comparable kinetics of plasma Hp consumption under baseline conditions. Ektacytometry data from blood samples from beagle dogs used in our study show a very similar rbc deformability/fragility as found in a human control population (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI39115DS1). This observation adds further value to the beagle dog model for investigating intravascular hemolysis. Additionally, the specific glucocorticoid responsiveness of the dog HP gene promotes the pharmacologic induction of supraphysiologic Hp plasma concentrations (21, 22). The dog is therefore a unique model, in which the effects of intravascular Hb can be analyzed at a range of targeted endogenous Hp plasma concentrations. The guinea pig, on the other hand, allowed us to analyze both vascular and extravascular effects of extracellular Hb in the presence or absence of exogenously administered, purified human Hp in a nonascorbate-producing species, with blood and tissue antioxidant status comparable to that of humans (30).
In our experiments, the hypertensive response in Hb-infused dogs similarly occurred at a plasma Hb concentration of more than 100 μM and reached a maximum level comparable to the canine model of intravascular hemolysis reported earlier (28). We can thus confirm that intravascular Hb infusion in dogs is a robust and reproducible system to study the acute cardiovascular toxicity of Hb at different plasma Hp levels. Modulation of local NO synthesis by glucocorticoids cannot be excluded as a Hp-independent mechanism that may blunt the Hb response in our prednisone-treated animals (31). However, the congruent observation made with pharmacologic Hp treatment in nonglucocorticoid-treated guinea pigs strongly suggests that overexpressed Hp is directly involved in limiting Hb-induced hypertension in dogs. To confirm that stable Hb complexation is an essential mechanism by which Hp can counter Hb toxicity in vivo, we examined the effects of Hp on hypertension mediated by chemically modified Hb, which is unable to bind Hp as a result of covalent α-globin subunit cross-linking (26). Our experiments clearly show that upon infusion into animals, αα-DBBF did not form a complex with Hp, and the hypertensive response was not attenuated, even in the presence of excess Hp. Based on data shown in Figure 3A, a complex multidimensional mechanism of blood pressure control appears to be at work. Since NO binding was not changed by Hb sequestration within the Hp complex, it is possible that the small blood pressure increase seen in guinea pigs transfused with Hb-Hp may be due to NO scavenging within the vascular compartment. However, it appears likely that yet-to-be-determined mechanisms of blood pressure control, regarding the Hb-Hp complex, are functioning.
Several mechanisms may contribute to Hb-mediated hypertension and vascular toxicity. NO consumption by the dioxygenase reaction of Hb, leading to a state of vascular depletion of NO, is the most widely investigated mechanism to date (32, 33). Under normal circumstances, the endothelial cell layer, rbc-free plasma zone, unstirred plasma layer around rbc, and erythrocyte membrane provide diffusion barriers, which impair rapid, unhindered interactions between rbc Hb and endothelial NO (34, 35). Upon hemolysis or infusion of cell-free Hb, these diffusion barriers are no longer effective in preventing free Hb from reaching and reacting with endothelial NO. Indeed, the vasoactivity of cell-free Hb blood substitutes is attributed to both NO scavenging and premature delivery of oxygen to systemic arterioles, both of which trigger vasoconstriction and subsequent elevation of blood pressure (36–40). Heme, the redox-active prosthetic group of Hb, generates and interacts with oxygen-free radicals (ROS), such as superoxide ion (O2•–) and H2O2 (41, 42). These radicals increase the vascular tone, either through direct modulation of vasoconstrictive pathways or inactivation of NO, providing another possible mechanism of Hb-induced vasoactivity (43–45). Hb-derived free radicals can potentially activate platelets, and markers of platelet activation were indeed found to be increased in patients with sickle-cell disease (46, 47). Platelet-released mediators like serotonin could therefore be another NO-independent cause of vascular complications in hemolytic anemias (48, 49). Following extensive examination of the effects of Hp complex formation on the redox and ligand-binding properties of Hb, we observed that neither NO dioxygenation, autoxidation, nor H2O2 redox cycling differed between free Hb and the Hb-Hp complex. Consistent with these in vitro observations, the level of methemoglobin (a form of Hb in which the iron molecules are in the Fe3+ state) was very similar in plasma of Hb-infused animals, regardless of their Hp plasma concentrations. In addition, oxygen binding to the Hb-Hp complex as well as its cooperativity were comparable to those of uncomplexed Hb. Thus, it appears unlikely that the reduced hypertensive activity of the Hb-Hp complex is solely due to altered ligand-binding or enzymatic properties of circulating Hb.
A possible mechanism that may have contributed to the control of vascular Hb effects in our models involves the molecular configuration of the complex, which can potentially hinder the transendothelial passage of Hb (50). An inverse, nonlinear relationship between Hb molecular size and vasoactivity exists in the case of chemically modified Hb, with a markedly reduced hypertensive effect of multitetrameric Hb compared with tetrameric Hb (51). This relationship, though controversial, may be attributed to the more restricted diffusion/transition of large (polymeric or conjugated) Hbs into the subendothelial space (38, 52, 53). Consistent with data obtained with chemically modified Hbs of different molecular sizes (50, 54), in our experiments, the most visible effect of Hb sequestration by Hp was the retention of infused Hb within the circulation, as reflected by the complete absence of renal Hb excretion in animals with high levels of endogenous or exogenously administered Hp. However, it is unlikely that the molecular weight of the Hb-Hp complex (>150 kDa) is the sole determining factor in preventing Hb-induced vasoactivity, since some larger polymeric Hbs with little or no tetramers induced a hypertensive response in animals and humans. Therefore, altered surface charge, Hp glycosylation patterns, and molecular shape of the complex may be other important factors contributing to the limited effect of the Hb-Hp complex on vascular tone. For instance, the rod-shaped Hb-Hp complex has a maximal molecular diameter of ~180–200 Å, corresponding to more than 6 times the diameter of the Hb tetramer (~30 Å) or more than 10 times the diameter of the Hb dimer (~15 Å) (55). A globular Hb polymer with an equivalent molecular diameter as the Hb-Hp complex would consist of approximately 30 Hb tetramers with a molecular weight of 2 MDa. Thus, considering all the available physiologic, biochemical, and structural evidence, we propose that compartmentalization of cell-free Hb within the intravascular space and away from extravascular spaces is a potential mechanism underlying Hp-induced attenuation of the hypertensive activity of Hb.
In addition to hypertension, oxidative stress–related tissue damage is a central mechanism of Hb-mediated toxicity (6). In mouse knockout models, the plasma Hb and heme scavengers, Hp and hemopexin, respectively, are essential for protection against heme-mediated oxidative damage (19, 56). While the redox reactions initiated at heme iron are well characterized from a chemical perspective, there is limited evidence that these reactions occur in vivo during hemolysis. Evaluating animal models of hemoglobinuria and exchange transfusion with a long circulating HBOC, we recently obtained evidence of globin and heme oxidative modifications, including oxidation of key β-chain amino acids and protein cross-linking, in animal blood and urine (9, 30, 57). Here, we found no evidence of heme oxidation within the circulation, as less than 2% of free intravascular Hb was observed in the oxidized Fe3+ state and no Hb was observed in the higher oxidation ferryl (Fe4+) state throughout the infusion time of up to 8 hours. However, in the absence of Hp, non-modified Hb can rapidly escape the intravascular compartment and might be exposed to more oxidant (or less antioxidant) environments within certain extravascular regions, such as the subendothelial space or the renal tubular system. Therefore, while Hp effectively restricts access of Hb to the extravascular compartment, it may also prevent the participation of Hb in potentially hazardous oxidative reactions within extravascular spaces. This is consistent with our observation that Hp is effective in preventing iron deposition and related lipid peroxidation (4-HNE reactivity) in renal tubular cells. The presence of Hb oxidation products in urine of non-Hp–treated guinea pigs indicates that peroxidative Hb reactions indeed occur within the kidneys of Hb-infused animals, and may be the actual source of iron accumulation and kidney oxidative damage in the absence of Hp.
In conclusion, our studies support the potential therapeutic application of Hp in preventing toxicities and end-organ injuries associated with circulating extracellular Hb. Alternatively, pharmacological stimulation of endogenous Hp synthesis or administration of Hp analogs that mimic Hb binding and the antioxidant properties of Hp are feasible strategies to attenuate Hb-mediated toxicity. High-dose glucocorticoid treatment has been shown to have beneficial effects in conditions associated with accelerated hemolysis. Glucocorticoids attenuate the severity of sickle-cell vaso-occlusive pain crisis, the acute chest syndrome, and the hyperhemolysis syndrome. It will be interesting to investigate in clinical studies whether enhanced Hp synthesis in these patients is among the mechanisms involved in the protective glucocorticoid effect (58–61). Additionally, our data demonstrate that the Hb-Hp complex retains oxygen-binding characteristics with prolonged circulating time. This presents an intriguing possibility that the Hb-Hp complex could be developed further as a safe and effective blood substitute.
-
Methods
Materials. Purified stroma and LPS-free Hb suspended in physiologic PBS at 6 g/dl (lot no. 9400-023) was a gift from Sangart Inc. αα-DBBF was provided by the United States Army. Mixed phenotype human Hp protein isolated from plasma and suspended in PBS at a concentration of 2 g/dl (designated Hp) was donated by Benesis Corporation. HES (6% HESpan) was purchased from B. Braun Medical Inc.
Animals and surgical preparation. All animal studies were approved by the Cantonal Veterinary Office (Zurich, Switzerland) and the US FDA/Center for Biologics Evaluation and Research Institutional Animal Care and Use Committee, and all experimental procedures were performed in adherence to the National Institutes of Health guidelines.
Beagle dogs. Purpose-bred male and female beagle dogs were used in all studies (18–30 months of age; mean body weight of 14.3 kg). Animals were randomized to receive individual treatment protocols, with an equal number of male and female dogs in each group. Dogs were kept in groups at the research unit of the Vetsuisse Faculty of the University of Zurich and fed a standard commercial maintenance pellet diet (Farmerdog; Kliba) once daily. Animals in the prednisone group were administered 4 mg/kg prednisone (twice daily) for 3 days, prior to the start of our experiments.
Guinea pigs. Animals (male Hartley) were purchased from Charles Rivers Laboratories and acclimatized for 1 week upon arrival at the animal care facility. All animals were fed normal diets (Teklad guinea pig chow; Harlan-Teklad) throughout the acclimatization period and weighed 350–450 g at the time of study. Catheterization of the right common carotid artery and left external jugular vein was performed as described previously (30).
Experimental animal protocols. A detailed description of the dog and guinea pig study experimental protocols can be found in the Supplemental Methods.
Plasma and urine Hb analysis. Spectral analysis of Hb and Hb-Hp in plasma samples and Hb in urine samples was performed using a HP-8453 rapid scanning diode array Spectrophotometer (Agilent Technologies). The concentrations of Hb Fe2+, Hb Fe3+, and hemichrome species were determined using multicomponent analysis, as reported previously (62). SEC and MALDI-MS were used to evaluate Hb binding to Hp in plasma and the presence Hb in urine under conditions previously described by our group (10, 30). MALDI-MS analysis was additionally employed to determine oxidative damage to Hb in urine (10).
Renal non-heme iron and lipid peroxidation. Non-heme Fe3+ iron was detected using Perl’s method, followed by DAB intensification (63). Lipid peroxidation immunohistochemistry was performed, using a mouse monoclonal antibody against 4-HNE–modified proteins (HNEJ-2) (Oxis International). Detailed staining protocols can be found in the Supplemental Methods. For quantitative image analysis, the total intensity of the positively stained areas was measured after setting respective color thresholds using SigmaScan Pro 5.0 (Systat Inc.).
Effects of Hp on biophysical properties and ligand interactions of Hb. Detailed experimental procedures for oxygen equilibrium studies, NO reaction kinetics, and autoxidation kinetics of Hb are provided in the Supplemental Methods.
Statistics. For the comparison of plasma Hb (heme) concentrations (conscious dogs) and MAP values (conscious and anesthetized dogs), AUC was determined for each individual animal. Mean group AUC values were then compared using 2-tailed Student’s t test. Two-tailed Student’s t test was also used to compare plasma Hp and urine Hb concentrations and for the comparison of quantitative image analysis data. For the comparison of plasma heme concentrations in the dose escalation protocol, linear regressions were calculated and slopes were compared using an F-test. All analyses were performed using GraphPad Prism version 5.01 (GraphPad Software Inc.). P values of less than 0.05 were considered statistically significant.
-
Acknowledgments
We are indebted to the Institute of Parasitology, Vetsuisse Faculty, Zurich for generously supporting the beagle dog studies and providing access to their animal research facility. We are particularly grateful to Armin Rudemann and Andrea Perreten for excellent technical help. This study was supported by the Swiss National Science Foundation (grant 31-120658), the Helmut Horten Foundation, and the Gianni Rubatto Foundation (all to D.J. Schaer) and by FDA/CBER Critical Path Funds (to A.I. Alayash). The findings and conclusions in this article have not been formally disseminated by the FDA and should not be construed to represent any agency determination or policy.
Address correspondence to: Dominik J. Schaer, Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland. Phone: 41-44-255-1111; Fax: 41-44-364-5339; E-mail: dominik.schaer@usz.ch. Or to: Abdu I. Alayash, CBER, FDA, NIH Building 29, Room 112, 8800 Rockville Pike, Bethesda, Maryland 20892, USA. Phone: (301) 827-3813; Fax: (301) 435-4034; E-mail: abdu.alayash@fda.hhs.gov.
-
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: αα-DBBF, Bis(3,5-dibromosalicyl)fumarate-cross-linked human Hb; Fe3+, ferric; Hb, hemoglobin; HBOC, Hb-based oxygen carrier; HES, Hetastarch; 4-HNE, 4-hydroxynonenal; Hp, haptoglobin; MALDI-MS, MALDI–mass spectrometry; MAP, mean arterial blood pressure; SEC, size exclusion chromatography; SFHb, stroma-free Hb.
Reference information: J. Clin. Invest.119:2271–2280 (2009). doi:10.1172/JCI39115
See the related article at Haptoglobin halts hemoglobin’s havoc.
-
References
- Rother, R.P., Bell, L., Hillmen, P., Gladwin, M.T. 2005. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 293:1653-1662.
- Gramaglia, I., et al. 2006. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat. Med. 12:1417-1422.
- Savage, W.J., Brodsky, R.A. 2007. New insights into paroxysmal nocturnal hemoglobinuria. Hematology. 12:371-376.
- Gladwin, M.T., Vichinsky, E. 2008. Pulmonary complications of sickle cell disease. N. Engl. J. Med. 359:2254-2265.
- Buehler, P.W., Alayash, A.I. 2008. All hemoglobin-based oxygen carriers are not created equally. Biochim. Biophys. Acta. 1784:1378-1381. View this article via: PubMed Google Scholar
- Alayash, A.I. 2004. Oxygen therapeutics: can we tame haemoglobin? Nat. Rev. Drug Discov. 3:152-159.
- Pamplona, A., Hanscheid, T., Epiphanio, S., Mota, M.M., Vigario, A.M. 2009. Cerebral malaria and the hemolysis/methemoglobin/heme hypothesis: Shedding new light on an old disease. Int. J. Biochem. Cell Biol. 41:711-716.
- Jia, Y., Buehler, P.W., Boykins, R.A., Venable, R.M., Alayash, A.I. 2007. Structural basis of peroxide-mediated changes in human hemoglobin: a novel oxidative pathway. J. Biol. Chem. 282:4894-4907.
- Buehler, P.W., et al. 2009. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood. 113:2578-86.
- Vallelian, F., et al. 2008. The reaction of hydrogen peroxide with hemoglobin induces extensive alpha-globin crosslinking and impairs the interaction of hemoglobin with endogenous scavenger pathways. Free Radic. Biol. Med. 45:1150-1158.
- Wood, K.C., Hsu, L.L., Gladwin, M.T. 2008. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic. Biol. Med. 44:1506-1528.
- Reiter, C.D., et al. 2002. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 8:1383-1389.
- Ascenzi, P., et al. 2005. Hemoglobin and heme scavenging. IUBMB Life. 57:749-759.
- Schaer, D.J., Alayash, A.I., Buehler, P.W. 2007. Gating the radical hemoglobin to macrophages: the anti-inflammatory role of CD163, a scavenger receptor. Antioxid. Redox. Signal. 9:991-999.
- Kristiansen, M., et al. 2001. Identification of the haemoglobin scavenger receptor. Nature. 409:198-201.
- Schaer, C.A., Vallelian, F., Imhof, A., Schoedon, G., Schaer, D.J. 2007. CD163-expressing monocytes constitute an endotoxin-sensitive Hb clearance compartment within the vascular system. J. Leukoc. Biol. 82:106-110.
- Schaer, D.J., et al. 2006. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 107:373-380.
- Wicher, K.B., Fries, E. 2006. Haptoglobin, a hemoglobin-binding plasma protein, is present in bony fish and mammals but not in frog and chicken. Proc. Natl. Acad. Sci. U. S. A. 103:4168-4173.
- Fagoonee, S., et al. 2005. Plasma protein haptoglobin modulates renal iron loading. Am. J. Pathol. 166:973-983. View this article via: PubMed Google Scholar
- Lim, S.-K., et al. 1998. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood. 92:1870-1877. View this article via: PubMed Google Scholar
- Martinez-Subiela, S., Ginel, P.J., Ceron, J.J. 2004. Effects of different glucocorticoid treatments on serum acute phase proteins in dogs. Vet. Rec. 154:814-817. View this article via: PubMed Google Scholar
- Kim, H., Baumann, H. 1997. The carboxyl-terminal region of STAT3 controls gene induction by the mouse haptoglobin promoter. J. Biol. Chem. 272:14571-14579.
- Barnes, S., et al. 2008. High-resolution mass spectrometry analysis of protein oxidations and resultant loss of function. Biochem. Soc. Trans. 36:1037-1044.
- Doorn, J.A., Hurley, T.D., Petersen, D.R. 2006. Inhibition of human mitochondrial aldehyde dehydrogenase by 4-hydroxynon-2-enal and 4-oxonon-2-enal. Chem. Res. Toxicol. 19:102-110.
- Ohshiro, T.U., Mukai, K., Kosaki, G. 1980. Prevention of hemoglobinuria by administration of haptoglobin. Res. Exp. Med. (Berl.). 177:1-12.
- Buehler, P.W., et al. 2008. Structural stabilization in tetrameric or polymeric hemoglobin determines its interaction with endogenous antioxidant scavenger pathways. Antioxid. Redox. Signal. 10:1449-1462.
- Wang, X., et al. 2004. Biological activity of nitric oxide in the plasmatic compartment. Proc. Natl. Acad. Sci. U. S. A. 101:11477-11482.
- Minneci, P.C., et al. 2005. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J. Clin. Invest. 115:3409-3417.
- Martinez-Subiela, S., Tecles, F., Ceron, J.J. 2003. Critical differences of acute phase proteins in canine serum samples. Vet. J. 166:233-237.
- Buehler, P.W., D’Agnillo, F., Hoffman, V., Alayash, A.I. 2007. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J. Pharmacol. Exp. Ther. 323:49-60.
- Wallerath, T., et al. 1999. Down-regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension. Proc. Natl. Acad. Sci. U. S. A. 96:13357-13362.
- Morris, C.R. 2008. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematology Am. Soc. Hematol. Educ. Program. 2008:177-185. View this article via: PubMed Google Scholar
- Doherty, D.H., et al. 1998. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat. Biotechnol. 16:672-676.
- Liao, J.C., Hein, T.W., Vaughn, M.W., Huang, K.T., Kuo, L. 1999. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 96:8757-8761.
- Vaughn, M.W., Huang, K.T., Kuo, L., Liao, J.C. 2000. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J. Biol. Chem. 275:2342-2348.
- Alayash, A.I., D’Agnillo, F., Buehler, P.W. 2007. First-generation blood substitutes: what have we learned? Biochemical and physiological perspectives. Expert Opin. Biol. Ther. 7:665-675.
- Yu, B., et al. 2008. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 117:1982-1990.
- Tsai, A.G., et al. 2006. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood. 108:3603-3610.
- Natanson, C., Kern, S.J., Lurie, P., Banks, S.M., Wolfe, S.M. 2008. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 299:2304-2312.
- Rohlfs, R.J., et al. 1998. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J. Biol. Chem. 273:12128-12134.
- Misra, H.P., Fridovich, I. 1972. The generation of superoxide radical during the autoxidation of hemoglobin. J. Biol. Chem. 247:6960-6962. View this article via: PubMed Google Scholar
- Sadrzadeh, S.M., Graf, E., Panter, S.S., Hallaway, P.E., Eaton, J.W. 1984. Hemoglobin. A biologic fenton reagent. J. Biol. Chem. 259:14354-14356. View this article via: PubMed Google Scholar
- Knock, G.A., et al. 2009. Superoxide constricts rat pulmonary arteries via Rho-kinase-mediated Ca(2+) sensitization. Free Radic. Biol. Med. 46:633-642.
- Just, A., Olson, A.J., Whitten, C.L., Arendshorst, W.J. 2007. Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide. Am. J. Physiol. Heart Circ. Physiol. 292:H83-H92.
- Just, A., Whitten, C.L., Arendshorst, W.J. 2008. Reactive oxygen species participate in acute renal vasoconstrictor responses induced by ETA and ETB receptors. Am. J. Physiol. Renal Physiol. 294:F719-F728.
- Villagra, J., et al. 2007. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 110:2166-2172.
- Handin, R.I., Karabin, R., Boxer, G.J. 1977. Enhancement of platelet function by superoxide anion. J. Clin. Invest. 59:959-965.
- Eddahibi, S., et al. 2001. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J. Clin. Invest. 108:1141-1150.
- Golino, P., et al. 1994. Local effect of serotonin released during coronary angioplasty. N. Engl. J. Med. 330:523-528.
- Nakai, K., et al. 1998. Permeability characteristics of hemoglobin derivatives across cultured endothelial cell monolayers. J. Lab. Clin. Med. 132:313-319.
- Olson, J.S., et al. 2004. NO scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic. Biol. Med. 36:685-697.
- Matheson, B., Kwansa, H.E., Bucci, E., Rebel, A., Koehler, R.C. 2002. Vascular response to infusions of a nonextravasating hemoglobin polymer. J. Appl. Physiol. 93:1479-1486. View this article via: PubMed Google Scholar
- Sakai, H., et al. 2000. Molecular dimensions of Hb-based O(2) carriers determine constriction of resistance arteries and hypertension. Am. J. Physiol. Heart Circ. Physiol. 279:H908-H915. View this article via: PubMed Google Scholar
- Matheson, B., Razynska, A., Kwansa, H., Bucci, E. 2000. Appearance of dissociable and cross-linked hemoglobins in the renal hilar lymph. J. Lab. Clin. Med. 135:459-464.
- Polticelli, F., Bocedi, A., Minervini, G., Ascenzi, P. 2008. Human haptoglobin structure and function — a molecular modelling study. FEBS J. 275:5648-5656. View this article via: PubMed Google Scholar
- Tolosano, E., et al. 1999. Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood. 94:3906-3914. View this article via: PubMed Google Scholar
- Manalo, D.J., et al. 2008. Acellular haemoglobin attenuates hypoxia-inducible factor-1alpha (HIF-1alpha) and its target genes in haemodiluted rats. Biochem. J. 414:461-469.
- Griffin, T.C., McIntire, D., Buchanan, G.R. 1994. High-dose intravenous methylprednisolone therapy for pain in children and adolescents with sickle cell disease. N. Engl. J. Med. 330:733-737.
- Bernini, J.C., et al. 1998. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood. 92:3082-3089. View this article via: PubMed Google Scholar
- Win, N., Yeghen, T., Needs, M., Chen, F.E., Okpala, I. 2004. Use of intravenous immunoglobulin and intravenous methylprednisolone in hyperhaemolysis syndrome in sickle cell disease. Hematology. 9:433-436.
- Strouse, J.J., Takemoto, C.M., Keefer, J.R., Kato, G.J., Casella, J.F. 2008. Corticosteroids and increased risk of readmission after acute chest syndrome in children with sickle cell disease. Pediatr. Blood Cancer. 50:1006-1012.
- Winterbourn, C.C. 1985. Reactions of superoxide with hemoglobin. InHandbook of methods of oxygen radical research. R.A. Greenwald, editor. CRC Press. Boca Raton, Florida, USA. 137–141.
- Meguro, R., et al. 2007. Nonheme-iron histochemistry for light and electron microscopy: a historical, theoretical and technical review. Arch. Histol. Cytol. 70:1-19.
.
View this article via: PubMed Google Scholar
-
Version history
- Version 1 (July 20, 2009): No description
- Version 2 (August 3, 2009): No description



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)








