Advertisement
Commentary Free access | 10.1172/JCI18233
Peanut allergy: a growing phenomenon
Wesley Burks
University of Arkansas for Medical Sciences, Arkansas Children’s Hospital, Little Rock, Arkansas, USA
Address correspondence to: Wesley Burks, Department of Pediatrics, Division of Allergy and Immunology, Arkansas Children’s Hospital, 1120 Marshall Street, Little Rock, Arkansas 72202, USA. Phone: (501) 364-1060; Fax: (501) 364-3173; E-mail: burkswesley@uams.edu.
Find articles by Burks, W. in: PubMed | Google Scholar
Published April 1, 2003 - More info
J Clin Invest. 2003;111(7):950–952. https://doi.org/10.1172/JCI18233.
© 2003 The American Society for Clinical Investigation
Peanut allergy is one of the most serious of the immediate hypersensitivity reactions to foods in terms of persistence and severity and appears to be a growing problem (1). The prevalence of food hypersensitivity in adults is reportedly less common, but a recent survey in the US found that 1.3% of adults are allergic to peanuts or tree nuts (2). Recently, in a cohort of American children referred for the evaluation of atopic dermatitis, the prevalence of allergic reactivity to peanuts was nearly twice as high as that in a similar group evaluated a decade earlier (3). In spite of increased recognition and understanding of food allergies, food is the single most common cause of anaphylaxis seen in hospital emergency departments (4), accounting for about one-third of anaphylaxis cases seen. It is estimated that about 30,000 food-induced anaphylactic events are seen in American emergency departments each year, 200 of which are fatal (5). Either peanuts or tree nuts cause more than 80% of these reactions.
Due to the persistence of the reaction and the lack of effective treatment, peanut-specific immunotherapy is currently being examined as a treatment option. An understanding of the molecular mechanisms is vital to ensure the eventual, effective treatment of peanut-allergic patients.
-
Th2 polarization of cytokines
The study by Turcanu et al. (6) in this issue of the JCI uses a novel approach, that of CFSE staining to separate peanut-specific lymphocytes by flow cytometry and subsequent cloning. Due to technical difficulties arising from the low frequency of allergen-specific cells in the blood, previous studies have been complicated and not easily interpreted. Human T cells, when repeatedly stimulated in vitro, will often develop a Th2 phenotype regardless of their origin (7, 8).
In this study, the authors compare peanut-allergic individuals, individuals who have outgrown peanut allergy, and those who had always tolerated peanuts (6). Peripheral blood lymphocytes in the peanut-allergic individuals demonstrated a Th2 polarization of cytokine production by peanut-specific cells with low levels of IFN-γ and TNF-α and high levels of IL-4, IL-5, and IL-13. In the individuals who had outgrown their peanut allergy and the nonallergic controls, the cytokines showed a Th1 bias with high levels of IFN-γ and TNF-α and low levels of IL-4, IL-5, and IL-13. An interesting part of the study investigated individuals who were allergic to eggs, using similar laboratory and cloning techniques. As in the peanut-specific cells, the egg-allergic individuals’ peripheral blood lymphocytes had a Th2 skewing of their ovalbumin-specific T cells (Figure 1).
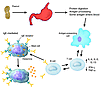 Figure 1
Figure 1Foods enter the gastrointestinal tract and undergo protein digestion and then antigen (Ag) processing. Some Ag enters the blood stream to be distributed at distal sites throughout the body (particularly the skin and respiratory mucosa along with sites in the gastrointestinal mucosa). As the antigen-presenting cell presents Ag to the T cell, specific cytokines are produced. In the peanut-allergic individual, the T cells will secrete increased amounts of IL-4, IL-5, and IL-13, among other mediators, and reduced amounts of IFN-γ and TNF-α when compared to that in an individual who is not allergic to peanuts. The T cell in turn regulates eventual peanut-specific IgE production by B cells. Peanut-specific IgE is attached to mast cells in the gastrointestinal tract, skin, and respiratory tract mucosa. Subsequently, on ingestion of peanuts, in the peanut-allergic individual, the protein is digested and the Ag binds peanut-specific IgE on the mast cell, causing activation of the mast cell with mediator release at the mucosal site. Clinical symptoms ensue.
This evidence that peanut antigens do not in themselves induce a Th2 cytokine response is not surprising, since all food allergy patients share similar clinical symptoms in the skin, gastrointestinal tract, and respiratory tract. The results of the present study (6) will help in the evaluation of future immunomodulatory treatments for food allergy and in studies that examine the natural history of food allergic reactions.
-
Peanut-specific IgE
The sequence of events necessary to produce allergen-specific IgE suggests several potential avenues for immunomodulatory intervention. These approaches can be placed into two general categories: (a) strategies designed to inhibit IgE or the interaction of mediators important to IgE production, and (b) strategies designed to alter T cell activation. The second category includes induction of T cell anergy by use of allergen-specific peptides (T cell epitopes), and modulation of Th cell development by means that favor a Th1 cytokine response and/or by prevention of T cell proliferation.
Allergen immunotherapy is an effective therapeutic modality to prevent further anaphylactic episodes in patients with insect-sting hypersensitivity when they have experienced significant systemic symptoms (9). Because allergen immunotherapy can downregulate the specific-IgE response and the cellular response to allergens, it is now being studied as a possible option for patients with lifelong food allergies (10). The goal of peanut-specific immunotherapy would not be to allow patients to eat peanuts at will, but rather to give them some protection in case of accidental ingestion. Given the partial rate of response and high rate of adverse reactions to traditional food immunotherapy in previous studies, and the frequent occurrence of accidental peanut ingestion, alternative forms of immunotherapy are necessary for this potentially fatal allergy. The findings by Turcanu et al. (6) provide additional laboratory data about the possible mechanism of the food hypersensitivity response that can be studied as patients with food allergy are placed on food-specific immunotherapy.
One of the more exciting recent developments in the treatment of food allergy is novel immunotherapeutic strategies designed to alter the immune system’s response to food allergens. These strategies are now being examined in animal models as potential treatment modalities (11). They include cytokine-modulated immunotherapy, immunostimulatory sequence–modulated immunotherapy, plasmid DNA immunotherapy, allergen-peptide immunotherapy, and “engineered” (mutated) allergen protein immunotherapy. All of these methods strive to elicit a Th1-type response or tolerance from the immune system in response to a specific food allergen.
-
Natural history of peanut allergy
It will be even be more interesting to elucidate why only a few food allergens induce the type of Th2 response early in life that is demonstrated in this study (6), and why certain people outgrow their food allergy. In this study, individuals allergic to peanuts and individuals allergic to eggs had a similar Th2-skewed cytokine response. However, the natural history of the clinical symptoms of peanut allergy is quite different from that of the symptoms of egg allergy. Peanut allergy is infrequently outgrown (12), while egg allergy is typically outgrown in the first 4–5 years of life.
The novel approach used by these authors to identify antigen-specific T cells will likely have important implications for future studies of allergic disease, as well as autoimmune disorders.
-
Footnotes
See the related article beginning on page 1065.
Conflict of interest: The author owns stock and/or stock options in SEER Inc.
-
References
- Sampson, HA. Clinical practice. Peanut allergy. N. Engl. J. Med. 2002. 346:1294-1299.
- Sicherer, SH, Munoz-Furlong, A, Burks, AW, Sampson, HA. Prevalence of peanut and tree nut allergy in the US determined by a random digit dial telephone survey. J. Allergy Clin. Immunol. 1999. 103:559-562.
- Sampson, HA. Managing peanut allergy. BMJ. 1996. 312:1050-1051. View this article via: PubMed Google Scholar
- Yocum, MW, et al. Epidemiology of anaphylaxis in Olmsted County: a population-based study. J. Allergy Clin. Immunol. 1999. 104:452-456.
- Bock, SA, Munoz-Furlong, A, Sampson, HA. Fatalities due to anaphylactic reactions to foods. J. Allergy Clin. Immunol. 2001. 107:191-193.
- Turcanu, V, Maleki, SJ, Lack, G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J. Clin. Invest. 2003. 111:1065-1072. doi:10.1172/JCI200316142.
- Cohen, SB, Webb, LM, Feldmann, M. The method of deriving human T-cell clones alters the proportion of IL-10-producing cells. Immunology. 1996. 87:343-347.
- Demeure, CE, et al. Human naive CD4 T cells produce interleukin-4 at priming and acquire a Th2 phenotype upon repetitive stimulations in neutral conditions. Eur. J. Immunol. 1995. 25:2722-2725.
- Valentine, MD, et al. The value of immunotherapy with venom in children with allergy to insect stings. N. Engl. J. Med. 1990. 323:1601-1603. View this article via: PubMed Google Scholar
- Oppenheimer, JJ, Nelson, HS, Bock, SA, Christensen, F, Leung, DY. Treatment of peanut allergy with rush immunotherapy. J. Allergy Clin. Immunol. 1992. 90:256-262.
- Burks, AW, Bannon, GA, Lehrer, SB. Classic specific immunotherapy and new perspectives in specific immunotherapy for food allergy. Allergy. 2001. 67:121-124.
- Skolnick, HS, et al. The natural history of peanut allergy. J. Allergy Clin. Immunol. 2001. 107:367-374.
-
Version history
- Version 1 (April 1, 2003): No description



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)

