Figure 1
Options:
View larger image
(or click on image)
Download as PowerPoint
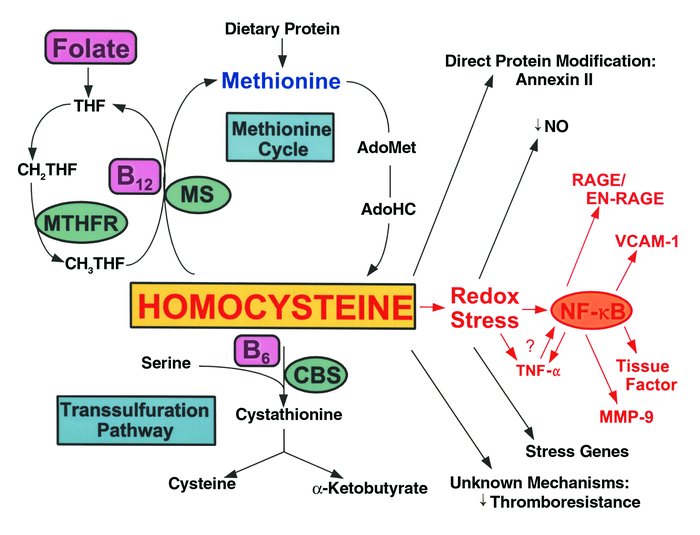
HC metabolism and vascular dysfunction. HC is formed upon demethylation of methionine via S-adenosylmethionine (AdoMet) and S-adenosylhomocysteine (AdoHC). HC is eliminated in the methionine cycle by remethylation through the action of methionine synthase (MS), a vitamin B12–dependent enzyme. In this reaction, methyltetrahydrofolate (CH3THF) serves as the methyl donor, and is formed from methylene tetrahydrofolate (CH2THF) through the action of methyltetrahydrofolate reductase (MTHFR). CH2THF is formed from tetrahydrofolate (THF), a folate derivative. Alternatively, HC can participate in transsulfuration in which it condenses with serine through the action of the vitamin B6-dependent enzyme, cystathionine-β-synthase (CBS). Cystathionine then splits into cysteine and α-ketobutyrate. HC can modify vascular cell function by forming a direct disulfide protein derivative, as in the case of the fibrinolytic receptor, annexin II; by inducing a prothrombotic phenotype through largely unknown mechanisms; or by undergoing auto-oxidation generating superoxide radicals (redox stress), leading to depletion of nitric oxide (NO•) and expression of acute stress–related genes. Redox stress may also activate the proinflammatory transcription factor NF-κB, which may induce expression of TNF-α, RAGE/EN-RAGE, VCAM-1, tissue factor, and MMP-9. TNF-α may also induce activation and nuclear translocation of NF-κB.



Copyright © 2025 American Society for Clinical Investigation
ISSN: 0021-9738 (print), 1558-8238 (online)